A new class of leukotriene biosynthesis inhibitor: the development of MK-0591.
P Prasit, M Belley, M Blouin, C Brideau, C Chan, S Charleson, J F Evans, R Frenette, J Y Gauthier, J Guay
Index: J. Lipid Mediat. 6(1-3) , 239-44, (1993)
Full Text: HTML
Abstract
The evolution of MK-0591 (3-[1-(4-chlorobenzyl)-3-(t-butylthio)-5-(quinolin-2-ylmethoxy+ ++)indol-2-yl]- 2,2-dimethylpropanoic acid), 12, a potent, orally active leukotriene biosynthesis inhibitor is described. MK-0591 is currently undergoing clinical evaluation as a potential agent for the treatment of asthma and inflammatory bowel disease. It acts through a novel mechanism by a specific interaction with a membrane protein, 5-lipoxygenase activating protein (FLAP), which has been shown to be essential for LT synthesis in inflammatory cells. A brief comparison of its biological activity with that of its progenitors MK-886 and L-674,636 is described.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
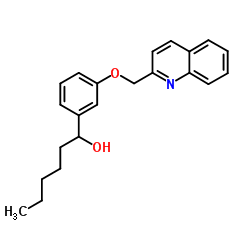 |
L-655238
CAS:101910-24-1 |
C22H25NO2 |
|
Early vascular permeability in murine experimental peritonit...
2002-04-01 [Inflammation 26(2) , 61-71, (2002)] |
|
Actions of cysteinyl leukotrienes in the enteric nervous sys...
2003-01-10 [Eur. J. Pharmacol. 459(1) , 27-39, (2003)] |
|
Enhanced activity of Ca2+-activated K+ channels by 1-[2-hydr...
2002-08-01 [J. Cell Physiol. 192(2) , 188-99, (2002)] |
|
Control of chondrocyte regulatory volume decrease (RVD) by [...
2008-03-01 [Osteoarthr. Cartil. 16(3) , 312-22, (2008)] |
|
Effect of a 5-lipoxygenase inhibitor and leukotriene antagon...
1992-12-01 [Br. J. Pharmacol. 107(4) , 1108-15, (1992)] |