L-655238
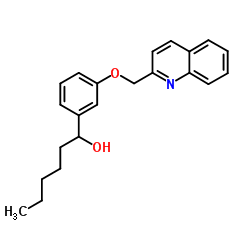
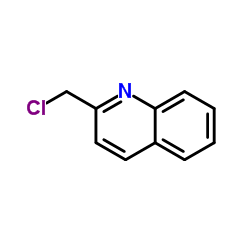
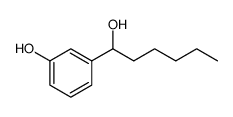
L-655238 structure
|
Common Name | L-655238 | ||
|---|---|---|---|---|
| CAS Number | 101910-24-1 | Molecular Weight | 335.439 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 497.7±35.0 °C at 760 mmHg | |
| Molecular Formula | C22H25NO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 254.8±25.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of L-655238REV 5901 is a competitive and orally active antagonist of leukotriene receptor, with a Ki of 0.7 μM. REV 5901 is also a 5-lipoxygenase inhibitor. REV 5901 can be used for the research of asthma in which leukotriene release be involved[1][2]. |
| Name | 1-[3-(quinolin-2-ylmethoxy)phenyl]hexan-1-ol |
|---|---|
| Synonym | More Synonyms |
| Description | REV 5901 is a competitive and orally active antagonist of leukotriene receptor, with a Ki of 0.7 μM. REV 5901 is also a 5-lipoxygenase inhibitor. REV 5901 can be used for the research of asthma in which leukotriene release be involved[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.7 μM (leukotriene receptor)[1] [2] |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 497.7±35.0 °C at 760 mmHg |
| Molecular Formula | C22H25NO2 |
| Molecular Weight | 335.439 |
| Flash Point | 254.8±25.9 °C |
| Exact Mass | 335.188538 |
| PSA | 42.35000 |
| LogP | 4.94 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.606 |
| InChIKey | JRLOEMCOOZSCQP-UHFFFAOYSA-N |
| SMILES | CCCCCC(O)c1cccc(OCc2ccc3ccccc3n2)c1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
|
~85% 
L-655238 CAS#:101910-24-1 |
| Literature: White; Yager; Stappenbeck Journal of Organic Chemistry, 1993 , vol. 58, # 12 p. 3466 - 3468 |
|
~% 
L-655238 CAS#:101910-24-1 |
| Literature: White; Yager; Stappenbeck Journal of Organic Chemistry, 1993 , vol. 58, # 12 p. 3466 - 3468 |
|
~% 
L-655238 CAS#:101910-24-1 |
| Literature: White; Yager; Stappenbeck Journal of Organic Chemistry, 1993 , vol. 58, # 12 p. 3466 - 3468 |
|
~% 
L-655238 CAS#:101910-24-1 |
| Literature: Musser; Chakraborty; Sciortino; Gordon; Khandwala; Neiss; Pruss; Van Inwegen; Weinryb; Coutts Journal of Medicinal Chemistry, 1987 , vol. 30, # 1 p. 96 - 104 |
|
~% 
L-655238 CAS#:101910-24-1 |
| Literature: Musser; Chakraborty; Sciortino; Gordon; Khandwala; Neiss; Pruss; Van Inwegen; Weinryb; Coutts Journal of Medicinal Chemistry, 1987 , vol. 30, # 1 p. 96 - 104 |
|
~% 
L-655238 CAS#:101910-24-1 |
| Literature: Musser; Chakraborty; Sciortino; Gordon; Khandwala; Neiss; Pruss; Van Inwegen; Weinryb; Coutts Journal of Medicinal Chemistry, 1987 , vol. 30, # 1 p. 96 - 104 |
|
~% 
L-655238 CAS#:101910-24-1 |
| Literature: White; Yager; Stappenbeck Journal of Organic Chemistry, 1993 , vol. 58, # 12 p. 3466 - 3468 |
|
~% 
L-655238 CAS#:101910-24-1 |
| Literature: White; Yager; Stappenbeck Journal of Organic Chemistry, 1993 , vol. 58, # 12 p. 3466 - 3468 |
|
Early vascular permeability in murine experimental peritonitis is co-mediated by resident peritoneal macrophages and mast cells: crucial involvement of macrophage-derived cysteinyl-leukotrienes.
Inflammation 26(2) , 61-71, (2002) The initial phase of zymosan-induced peritonitis involves an increase of vascular permeability (peak at 30 min) that is correlated with high levels of vasoactive eicosanoids, namely, prostaglandins (P... |
|
|
Actions of cysteinyl leukotrienes in the enteric nervous system of guinea-pig stomach and small intestine.
Eur. J. Pharmacol. 459(1) , 27-39, (2003) Conventional intracellular microelectrodes, neuronal tracer injection techniques and immunohistochemistry were used to study the actions of cysteinyl leukotrienes (CysLTs) on electrical and synaptic b... |
|
|
Enhanced activity of Ca2+-activated K+ channels by 1-[2-hydroxy-3-propyl-4-[(1H-tetrazol-5-yl)butoxyl]phenyl] ethanone (LY-171883) in neuroendocrine and neuroblastoma cell lines.
J. Cell Physiol. 192(2) , 188-99, (2002) The effects of LY-171883, an orally active leukotriene antagonist, on membrane currents were examined in pituitary GH(3) and in neuroblastoma IMR-32 cells. In GH(3) cells, LY-171883 (1-300 microM) rev... |
| rev-5901 |
| rg 5901 |
| Benzenemethanol, α-pentyl-3-(2-quinolinylmethoxy)- |
| 1-[3-(2-Quinolinylmethoxy)phenyl]-1-hexanol |
| pf 5901 |
| 1-[3-(Quinolin-2-ylmethoxy)phenyl]hexan-1-ol |