Synthesis and biological evaluation of epothilone A dimeric compounds
Daniele Passarella, Daniela Comi, Graziella Cappelletti, Daniele Cartelli, Juerg Gertsch, Ana R. Quesada, Jurgen Borlak, Karl-Heinz Altmann, Daniele Passarella, Daniela Comi, Graziella Cappelletti, Daniele Cartelli, Juerg Gertsch, Ana R. Quesada, Jurgen Borlak, Karl-Heinz Altmann
Index: Bioorg. Med. Chem. 17 , 7435-40, (2009)
Full Text: HTML
Abstract
The preparation and biological evaluation of a novel series of dimeric epothilone A derivatives (1-6) are described. Two types of diacyl spacers were introduced to establish the various dimeric epothilone A constructs. The effect of these compounds on tubulin polymerization and their cytotoxicity against four different cancer cell lines are reported. Several of the newly synthesized compounds inhibit endothelial cell differentiation and endothelial cell migration that are key steps of the angiogenic process.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
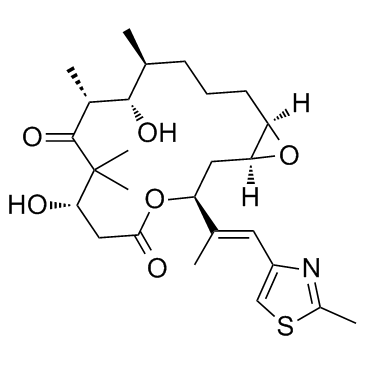 |
Epothilone A
CAS:152044-53-6 |
C26H39NO6S |
|
Identifying off-target effects and hidden phenotypes of drug...
2006-06-01 [Nat. Chem. Biol. 2 , 329-37, (2006)] |
|
Neuronal transcriptional repressor REST suppresses an Atoh7-...
2011-01-01 [Dev. Biol. 349 , 90-9, (2011)] |
|
Conformational preferences of natural and C3-modified epothi...
2008-03-13 [J. Med. Chem. 51 , 1469-73, (2008)] |
|
Cyclostreptin binds covalently to microtubule pores and lume...
2007-02-01 [Nat. Chem. Biol. 3 , 117-25, (2007)] |
|
Synthesis and SAR of C12-C13-oxazoline derivatives of epothi...
2009-07-15 [Bioorg. Med. Chem. Lett. 19 , 3760-3, (2009)] |