Epothilone A
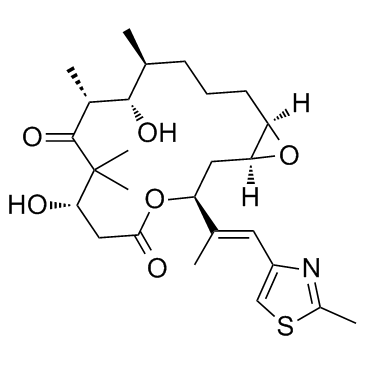
Epothilone A structure
|
Common Name | Epothilone A | ||
|---|---|---|---|---|
| CAS Number | 152044-53-6 | Molecular Weight | 493.656 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 683.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C26H39NO6S | Melting Point | 95ºC | |
| MSDS | Chinese USA | Flash Point | 367.1±31.5 °C | |
Use of Epothilone AEpothilone A is a competitive inhibitor of the binding of [3H] paclitaxel to tubulin polymers, with a Ki of 0.6-1.4 μM. |
| Name | (1R,5S,6S,7R,10S,14S,16S)-6,10-dihydroxy-5,7,9,9-tetramethyl-14-[(E)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-13,17-dioxabicyclo[14.1.0]heptadecane-8,12-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Epothilone A is a competitive inhibitor of the binding of [3H] paclitaxel to tubulin polymers, with a Ki of 0.6-1.4 μM. |
|---|---|
| Related Catalog | |
| In Vitro | Epothilone A is a competitive inhibitor of the binding of [3H] paclitaxel to tubulin polymers. The apparent Ki value for Epothilone A is 1.4 μM by Hanes analysis and 0.6 μM by Dixon analysis[1]. Epothilone A, is noted to be highly cytotoxic (IC50=0.05 μM) in vitro when applied to the human T-24 bladder carcinoma cell line. The binding affinity of Epothilone A to tubulin is of the same order of magnitude as the binding affinity of paclitaxel to tubulin based on competition assays. The IC50 for displacement of 100 nM of (3H) paclitaxel from the tubulin binding site is 3.6 μM for paclitaxel, 2.3 μM for Epothilone A, and 3.3 μM for patupilone[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 683.3±55.0 °C at 760 mmHg |
| Melting Point | 95ºC |
| Molecular Formula | C26H39NO6S |
| Molecular Weight | 493.656 |
| Flash Point | 367.1±31.5 °C |
| Exact Mass | 493.249817 |
| PSA | 137.49000 |
| LogP | 2.54 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.532 |
| InChIKey | HESCAJZNRMSMJG-KKQRBIROSA-N |
| SMILES | CC(=Cc1csc(C)n1)C1CC2OC2CCCC(C)C(O)C(C)C(=O)C(C)(C)C(O)CC(=O)O1 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
Identifying off-target effects and hidden phenotypes of drugs in human cells.
Nat. Chem. Biol. 2 , 329-37, (2006) We present a strategy for identifying off-target effects and hidden phenotypes of drugs by directly probing biochemical pathways that underlie therapeutic or toxic mechanisms in intact, living cells. ... |
|
|
Neuronal transcriptional repressor REST suppresses an Atoh7-independent program for initiating retinal ganglion cell development.
Dev. Biol. 349 , 90-9, (2011) As neuronal progenitors differentiate into neurons, they acquire a unique set of transcription factors. The transcriptional repressor REST prevents progenitors from undergoing differentiation. Notably... |
|
|
Conformational preferences of natural and C3-modified epothilones in aqueous solution.
J. Med. Chem. 51 , 1469-73, (2008) The conformational properties of the microtubule-stabilizing agent epothilone A ( 1a) and its 3-deoxy and 3-deoxy-2,3-didehydro derivatives 2 and 3 have been investigated in aqueous solution by a comb... |
| UNII-51E07YBX96 |
| Epothilone A |
| (-)-Epothilone A |
| Epo A |
| 4,17-Dioxabicyclo[14.1.0]heptadecane-5,9-dione, 7,11-dihydroxy-8,8,10,12-tetramethyl-3-[(E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-, (1S,3S,7S,10R,11S,12S,16R)- |
| [1R*,3R*(E),7R*,10S*,11R*,12R*,16S*]-7,11-dihydroxy-8,8,10,12-tetramethyl-3-[1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione |
| Epothilon A |
| (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12-tetramethyl-3-[(1E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione |
| (1S,3S,7S,10R,11S,12S,16R)-7,11-Dihydroxy-8,8,10,12-tetramethyl-3-[(1E)-1-(2-methyl-1,3-thiazol-4-yl)-1-propen-2-yl]-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione |
| 4,17-Dioxabicyclo[14.1.0]heptadecane-5,9-dione, 7,11-dihydroxy-8,8,10,12-tetramethyl-3-[(E)-1-methyl-2-(2-methyl-4-thiazolyl)ethenyl]-, (1R,3R,7R,10S,11R,12R,16S)- |
| (1R,3R,7R,10S,11R,12R,16S)-7,11-Dihydroxy-8,8,10,12-tetramethyl-3-[(1E)-1-(2-methyl-1,3-thiazol-4-yl)-1-propen-2-yl]-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione |
| epithilone A |
| Epothilones |
| (1S,3S,7S,10R,11S,12S,16R)-7,11-dihydroxy-8,8,10,12-tetramethyl-3-[(1E)-1-(2-methyl-1,3-thiazol-4-yl)prop-1-en-2-yl]-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione |
 CAS#:186692-73-9
CAS#:186692-73-9 CAS#:186692-68-2
CAS#:186692-68-2 CAS#:184246-38-6
CAS#:184246-38-6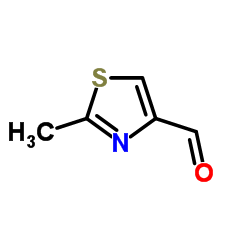 CAS#:20949-84-2
CAS#:20949-84-2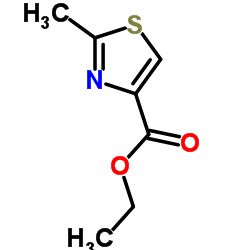 CAS#:6436-59-5
CAS#:6436-59-5 CAS#:187283-46-1
CAS#:187283-46-1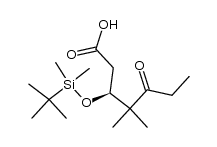 CAS#:187283-45-0
CAS#:187283-45-0