Epothilone A
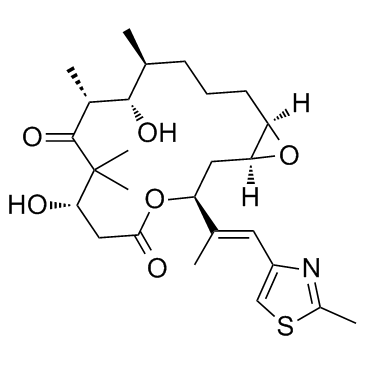
Epothilone A structure
|
Common Name | Epothilone A | ||
|---|---|---|---|---|
| CAS Number | 152044-53-6 | Molecular Weight | 493.656 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 683.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C26H39NO6S | Melting Point | 95ºC | |
| MSDS | Chinese USA | Flash Point | 367.1±31.5 °C | |
|
Identifying off-target effects and hidden phenotypes of drugs in human cells.
Nat. Chem. Biol. 2 , 329-37, (2006) We present a strategy for identifying off-target effects and hidden phenotypes of drugs by directly probing biochemical pathways that underlie therapeutic or toxic mechanisms in intact, living cells. High-content protein-fragment complementation assays (PCAs)... |
|
|
Neuronal transcriptional repressor REST suppresses an Atoh7-independent program for initiating retinal ganglion cell development.
Dev. Biol. 349 , 90-9, (2011) As neuronal progenitors differentiate into neurons, they acquire a unique set of transcription factors. The transcriptional repressor REST prevents progenitors from undergoing differentiation. Notably, REST binding sites are often associated with retinal gang... |
|
|
Conformational preferences of natural and C3-modified epothilones in aqueous solution.
J. Med. Chem. 51 , 1469-73, (2008) The conformational properties of the microtubule-stabilizing agent epothilone A ( 1a) and its 3-deoxy and 3-deoxy-2,3-didehydro derivatives 2 and 3 have been investigated in aqueous solution by a combination of NMR spectroscopic methods, Monte Carlo conformat... |
|
|
Cyclostreptin binds covalently to microtubule pores and lumenal taxoid binding sites.
Nat. Chem. Biol. 3 , 117-25, (2007) Cyclostreptin (1), a natural product from Streptomyces sp. 9885, irreversibly stabilizes cellular microtubules, causes cell cycle arrest, evades drug resistance mediated by P-glycoprotein in a tumor cell line and potently inhibits paclitaxel binding to microt... |
|
|
Synthesis and biological evaluation of epothilone A dimeric compounds
Bioorg. Med. Chem. 17 , 7435-40, (2009) The preparation and biological evaluation of a novel series of dimeric epothilone A derivatives (1-6) are described. Two types of diacyl spacers were introduced to establish the various dimeric epothilone A constructs. The effect of these compounds on tubulin... |
|
|
Synthesis and SAR of C12-C13-oxazoline derivatives of epothilone A.
Bioorg. Med. Chem. Lett. 19 , 3760-3, (2009) The SAR of a series of new epothilone A derivatives with a 2-substituted-1,3-oxazoline moiety trans-fused to the C12-C13 bond of the deoxy macrocycle have been investigated with regard to tubulin polymerization induction and cancer cell growth inhibition. Sig... |
|
|
Joys of molecules. 2. Endeavors in chemical biology and medicinal chemistry.
J. Med. Chem. 48 , 5613-38, (2005)
|