Piplartine
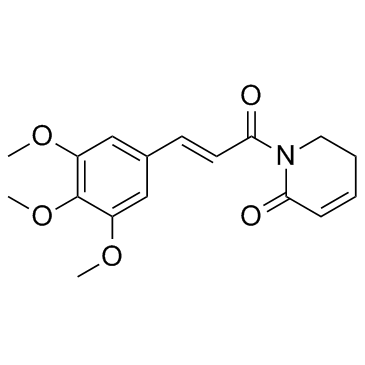
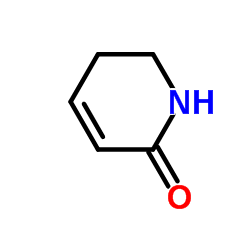
Piplartine structure
|
Common Name | Piplartine | ||
|---|---|---|---|---|
| CAS Number | 20069-09-4 | Molecular Weight | 317.336 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 475.6±45.0 °C at 760 mmHg | |
| Molecular Formula | C17H19NO5 | Melting Point | 124ºC | |
| MSDS | Chinese USA | Flash Point | 241.4±28.7 °C | |
Use of PiplartinePiperlongumine is a natural alkaloid isolated from Piper longum Linn[1], possesses ant-inflammatory, antibacterial, antiangiogenic, antioxidant, antitumor, and antidiabetic activities[2]. Piperlongumine induces ROS, and induces apoptosis in cancer cell lines[1]. Piperlongumine shows anti-cardiac fibrosis activity, suppresses myofibroblast transformation via suppression of the ERK1/2 signaling pathway[2]. |
| Name | Piperlongumine |
|---|---|
| Synonym | More Synonyms |
| Description | Piperlongumine is a natural alkaloid isolated from Piper longum Linn[1], possesses ant-inflammatory, antibacterial, antiangiogenic, antioxidant, antitumor, and antidiabetic activities[2]. Piperlongumine induces ROS, and induces apoptosis in cancer cell lines[1]. Piperlongumine shows anti-cardiac fibrosis activity, suppresses myofibroblast transformation via suppression of the ERK1/2 signaling pathway[2]. |
|---|---|
| Related Catalog | |
| Target |
ERK1 ERK2 |
| In Vitro | Piplartine (5, 10, and 15 μM) significantly decreases cell proliferation of 786-O, SKBR3, Panc1, A549, and L3.6pL cancer cells after treatment for 24 and 48 hours, induces apoptosis and ROS in these cell lines at 5 and 10 μM after 3 or 9 h of treatment[1]. Piplartine (5 or 10 μM) induces cleaved PARP and downregulates Sp1, Sp3, Sp4, and Sp-regulated genes[1]. Piplartine (20 μM) decreases the viability of cardiac fibroblasts (CFs). Piplartine (0-10 μM) suppresses myofibroblast transformation via suppression of the ERK1/2 signaling pathway[2]. |
| In Vivo | Piperlongumine (30 mg/kg/day, i.p. for 3 weeks) exhibits potent anti-tumor effect in athymic nude mice bearing L3.6pL cells without body weight loss[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 475.6±45.0 °C at 760 mmHg |
| Melting Point | 124ºC |
| Molecular Formula | C17H19NO5 |
| Molecular Weight | 317.336 |
| Flash Point | 241.4±28.7 °C |
| Exact Mass | 317.126312 |
| PSA | 65.07000 |
| LogP | 2.34 |
| Appearance of Characters | white to beige |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.581 |
| InChIKey | VABYUUZNAVQNPG-BQYQJAHWSA-N |
| SMILES | COc1cc(C=CC(=O)N2CCC=CC2=O)cc(OC)c1OC |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ≥5mg/mL at warmed to 60°C |
| RIDADR | NONH for all modes of transport |
|---|---|
| WGK Germany | 3 |
| RTECS | UU7785850 |
| HS Code | 2933399090 |
|
~42% 
Piplartine CAS#:20069-09-4 |
| Literature: Boll, Per M.; Hansen, Jesper; Simonsen, Ole; Thorup, Niels Tetrahedron, 1984 , vol. 40, # 1 p. 171 - 176 |
|
~% 
Piplartine CAS#:20069-09-4 |
| Literature: Tetrahedron, , vol. 40, # 1 p. 171 - 176 |
|
~% 
Piplartine CAS#:20069-09-4 |
| Literature: European Journal of Medicinal Chemistry, , vol. 57, p. 344 - 361 |
|
~% 
Piplartine CAS#:20069-09-4 |
| Literature: European Journal of Medicinal Chemistry, , vol. 57, p. 344 - 361 |
|
~% 
Piplartine CAS#:20069-09-4 |
| Literature: European Journal of Medicinal Chemistry, , vol. 57, p. 344 - 361 |
|
~% 
Piplartine CAS#:20069-09-4 |
| Literature: European Journal of Medicinal Chemistry, , vol. 57, p. 344 - 361 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease.
Brain 138 , 3221-37, (2015) Polyglutamine diseases are a class of dominantly inherited neurodegenerative disorders for which there is no effective treatment. Here we provide evidence that activation of serotonergic signalling is... |
|
|
Piperlongumine induces apoptotic and autophagic death of the primary myeloid leukemia cells from patients via activation of ROS-p38/JNK pathways.
Acta Pharmacol. Sin. 36(3) , 362-74, (2015) To investigate the effects of piperlongumine (PL), an anticancer alkaloid from long pepper plants, on the primary myeloid leukemia cells from patients and the mechanisms of action.Human BM samples wer... |
|
|
Piperlongumine for Enhancing Oral Bioavailability and Cytotoxicity of Docetaxel in Triple-Negative Breast Cancer.
J. Pharm. Sci. 104 , 4417-26, (2016) Very low oral bioavailability due to extensive pre-systemic metabolism and P-gp efflux has constrained the oral metronomic chemotherapy of docetaxel (DTX). There is tremendous need of compounds facili... |
|
Name: Fluorescence-based cell-based primary high throughput screening assay to identify ago...
Source: The Scripps Research Institute Molecular Screening Center
Target: muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id: CHRM1_AG_FLUO8_1536_1X%ACT PRUN
|
|
Name: Antitrypanosomal activity against epimastigote forms of Trypanosoma cruzi Y after 72 ...
Source: ChEMBL
Target: Trypanosoma cruzi
External Id: CHEMBL3059482
|
|
Name: Inhibition of MAPK/NFkappaB in ICR mouse RAW264.7 cells assessed as reduction in LPS-...
Source: ChEMBL
Target: N/A
External Id: CHEMBL4387890
|
|
Name: Fluorescence-based cell-based primary high throughput screening assay to identify pos...
Source: The Scripps Research Institute Molecular Screening Center
Target: muscarinic acetylcholine receptor M1 [Homo sapiens]
External Id: CHRM1_PAM_FLUO8_1536_1X%ACT PRUN
|
|
Name: Inhibition of MAPK/NFkappaB in ICR mouse RAW264.7 cells assessed as reduction in LPS-...
Source: ChEMBL
Target: N/A
External Id: CHEMBL4387892
|
|
Name: Cytotoxicity against ICR mouse RAW264.7 cells assessed as effect on cell proliferatio...
Source: ChEMBL
Target: RAW264.7
External Id: CHEMBL4387894
|
|
Name: Induction of autophagy in human MDA-MB-231 cells transfected with mRFP-eGFP-LC3 asses...
Source: ChEMBL
Target: MDA-MB-231
External Id: CHEMBL4773282
|
|
Name: Induction of autophagy in human MDA-MB-231 cells transfected with mRFP-eGFP-LC3 asses...
Source: ChEMBL
Target: MDA-MB-231
External Id: CHEMBL4773281
|
|
Name: Induction of autophagy in human MDA-MB-231 cells transfected with mRFP-eGFP-LC3 asses...
Source: ChEMBL
Target: MDA-MB-231
External Id: CHEMBL4773286
|
|
Name: Induction of apoptosis in human MDA-MB-231 cells at 1.8 uM measured after 72 hrs by A...
Source: ChEMBL
Target: MDA-MB-231
External Id: CHEMBL4773274
|
| Piperlongumine |
| 1-[(E)-3-(3,4,5-trimethoxyphenyl)prop-2-enoyl]-2,3-dihydropyridin-6-one |
| (E)-Piplartine |
| 1-[(2E)-3-(3,4,5-Trimethoxyphenyl)-2-propenoyl]-5,6-dihydro-2(1H)-pyridinone |
| piplartine |
| 1-[3-(3,4,5-Trimethoxy-phenyl)-acryloyl]-5,6-dihydro-1H-pyridin-2-one |
| 1-[(2E)-3-(3,4,5-Trimethoxyphenyl)prop-2-enoyl]-5,6-dihydropyridin-2(1H)-one |