3,4,5-Trimethoxy-trans-cinnamic acid
Modify Date: 2024-01-02 18:08:37
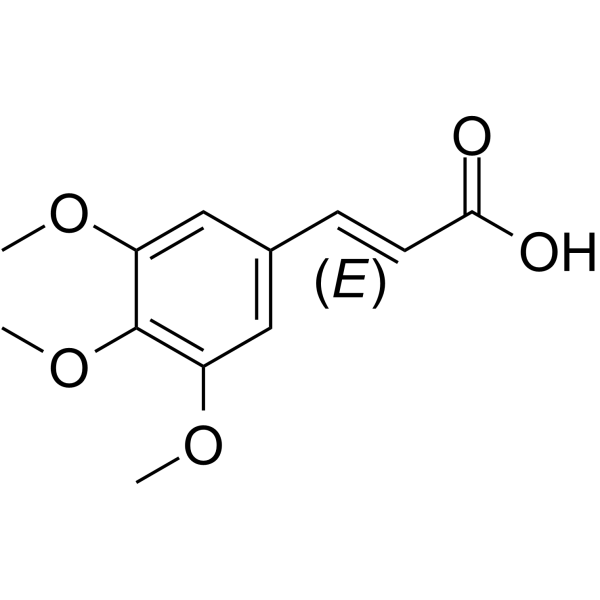
3,4,5-Trimethoxy-trans-cinnamic acid structure
|
Common Name | 3,4,5-Trimethoxy-trans-cinnamic acid | ||
|---|---|---|---|---|
| CAS Number | 20329-98-0 | Molecular Weight | 238.237 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 396.4±37.0 °C at 760 mmHg | |
| Molecular Formula | C12H14O5 | Melting Point | 125-127ºC(lit.) | |
| MSDS | N/A | Flash Point | 151.5±20.0 °C | |
Use of 3,4,5-Trimethoxy-trans-cinnamic acid(E)-3,4,5-Trimethoxycinnamic acid (TMCA) is a cinnamic acid substituted by multi-methoxy groups. (E)-3,4,5-Trimethoxycinnamic acid is an orally active and potent GABAA/BZ receptor agonist. (E)-3,4,5-Trimethoxycinnamic exhibits favourable binding affinity to 5-HT2C and 5-HT1A receptor, with IC50 values of 2.5 and 7.6 μM, respectively. (E)-3,4,5-Trimethoxycinnamic acid shows anticonvulsant and sedative activity. (E)-3,4,5-Trimethoxycinnamic acid can be used for the research of insomnia, headache and epilepsy[1][2][3]. |
| Name | 3-(3,4,5-trimethoxyphenyl)prop-2-enoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | (E)-3,4,5-Trimethoxycinnamic acid (TMCA) is a cinnamic acid substituted by multi-methoxy groups. (E)-3,4,5-Trimethoxycinnamic acid is an orally active and potent GABAA/BZ receptor agonist. (E)-3,4,5-Trimethoxycinnamic exhibits favourable binding affinity to 5-HT2C and 5-HT1A receptor, with IC50 values of 2.5 and 7.6 μM, respectively. (E)-3,4,5-Trimethoxycinnamic acid shows anticonvulsant and sedative activity. (E)-3,4,5-Trimethoxycinnamic acid can be used for the research of insomnia, headache and epilepsy[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | (E)-3,4,5-Trimethoxycinnamic acid (10 μg/mL, 1 h) increases the expressions of GAD65 and γ-subunit of GABAA receptors in the cerebellar granule cells[3]. (E)-3,4,5-Trimethoxycinnamic acid (0-10 μg/mL, 1 h) shows a significant increase in Cl- influx[3]. Western Blot Analysis[3] Cell Line: Primary cultured cerebellar granule cells Concentration: 10 μg/mL Incubation Time: 1 h Result: Increased expression of GAD65 (glutamic acid decarboxylase) and γ-subunit of GABAA receptors, but did not influence the amounts of a-, b-subunits in the GABAA receptors. Cell Viability Assay[3] Cell Line: Primary cultured cerebellar granule cells Concentration: 1, 3, 5, 10 μg/mL Incubation Time: 1 h Result: Produced a significant increase in Cl- influx. |
| In Vivo | (E)-3,4,5-Trimethoxycinnamic acid (0-20 mg/kg, IP, once) shows anti-seizure effects[2]. (E)-3,4,5-Trimethoxycinnamic acid (0-10 mg/kg, Orally, once) enhances hypnotic effects in pentobarbital-treated mice[3]. Animal Model: Ault male KunMing-strain mice (18-20 g, maximal electroshock (MES) and pentylenetetrazol (PTZ) models)[2] Dosage: 5, 10 and 20 mg/kg; 10 mL/kg Administration: IP, once Result: Significantly decreased the incidence of MES-induced THE (tonic hindlimb extension) to 50% and 20% of the value of the vehicle controls at 10 and 20 mg/kg. Decreased the incidence of MES-induced THE to only 80% at 5 mg/kg. Significantly delayed the onset of myoclonic jerks (MJ), and decreased the seizure severity and mortality compared with the vehicle-treated animals in PTZ seizure model. The incidence of generalized clonic convulsions (stage 4) disappeared at doses of both 10 and 20 mg/kg. Animal Model: ICR male mice (25-28 g, 10-12 in each group)[3] Dosage: 2, 5 and 10 mg/kg Administration: Orally (p.o.), once, 15 min and 1 h prior to pentobarbital injection Result: Significantly decreased locomotor activity at 10 mg/kg. Increased NREM and total sleep, but decreased wakefulness. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 396.4±37.0 °C at 760 mmHg |
| Melting Point | 125-127ºC(lit.) |
| Molecular Formula | C12H14O5 |
| Molecular Weight | 238.237 |
| Flash Point | 151.5±20.0 °C |
| Exact Mass | 238.084122 |
| PSA | 64.99000 |
| LogP | 2.00 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.560 |
| Safety Phrases | 24/25 |
|---|---|
| WGK Germany | 2 |
| RTECS | GE0722000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| O-Methylsinapic acid |
| 3,4,5-Trimethoxycinnamic_acid |
| (E)-6,7,8-trimethoxycinnamic acid |
| (2E)-3-(3,4,5-Trimethoxyphenyl)acrylic acid |
| 2-Propenoic acid, 3-(3,4,5-trimethoxyphenyl)-, (2E)- |
| 3,4,5-Trimethoxycinnamic acid |
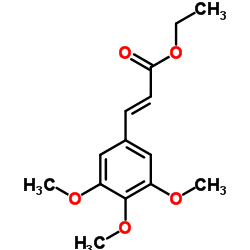 CAS#:1878-29-1
CAS#:1878-29-1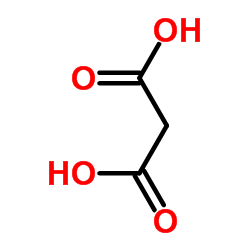 CAS#:141-82-2
CAS#:141-82-2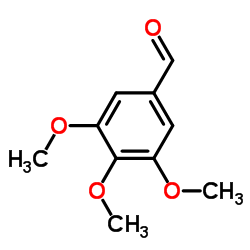 CAS#:86-81-7
CAS#:86-81-7 CAS#:682805-47-6
CAS#:682805-47-6 CAS#:1530-45-6
CAS#:1530-45-6 CAS#:530-59-6
CAS#:530-59-6 CAS#:77-78-1
CAS#:77-78-1 CAS#:3044-56-2
CAS#:3044-56-2 CAS#:186581-53-3
CAS#:186581-53-3 CAS#:82410-35-3
CAS#:82410-35-3![1,2,3-trimethoxy-5-[3-(3-prop-2-enoxyphenoxy)prop-1-enyl]benzene structure](https://image.chemsrc.com/caspic/314/104761-74-2.png) CAS#:104761-74-2
CAS#:104761-74-2![1,3-diethyl-7-methyl-8-[(E)-2-(3,4,5-trimethoxyphenyl)ethenyl]purine-2,6-dione structure](https://image.chemsrc.com/caspic/042/147700-40-1.png) CAS#:147700-40-1
CAS#:147700-40-1![1,3-dipropyl-8-[(E)-2-(3,4,5-trimethoxyphenyl)ethenyl]-7H-purine-2,6-dione structure](https://image.chemsrc.com/caspic/013/141807-97-8.png) CAS#:141807-97-8
CAS#:141807-97-8 CAS#:20069-09-4
CAS#:20069-09-4 CAS#:10263-19-1
CAS#:10263-19-1 CAS#:30687-11-7
CAS#:30687-11-7 CAS#:1703-35-1
CAS#:1703-35-1 CAS#:1504-56-9
CAS#:1504-56-9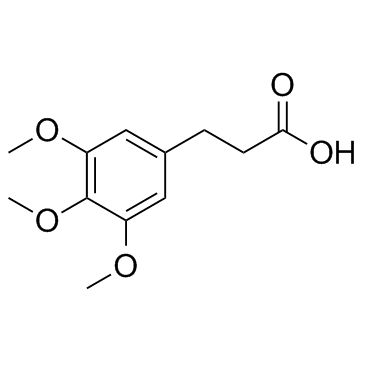 CAS#:25173-72-2
CAS#:25173-72-2
