L-1-Phenylethylamine
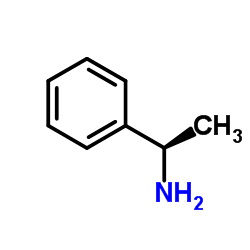
L-1-Phenylethylamine structure
|
Common Name | L-1-Phenylethylamine | ||
|---|---|---|---|---|
| CAS Number | 2627-86-3 | Molecular Weight | 121.180 | |
| Density | 0.94 | Boiling Point | 187-189 ºC | |
| Molecular Formula | C8H11N | Melting Point | -10ºC | |
| MSDS | Chinese USA | Flash Point | 79 ºC | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
| Name | (1S)-1-phenylethanamine |
|---|---|
| Synonym | More Synonyms |
| Density | 0.94 |
|---|---|
| Boiling Point | 187-189 ºC |
| Melting Point | -10ºC |
| Molecular Formula | C8H11N |
| Molecular Weight | 121.180 |
| Flash Point | 79 ºC |
| Exact Mass | 121.089149 |
| PSA | 26.02000 |
| LogP | 1.44 |
| Vapour Pressure | 0.8±0.3 mmHg at 25°C |
| Index of Refraction | 1.533 |
| InChIKey | RQEUFEKYXDPUSK-ZETCQYMHSA-N |
| SMILES | CC(N)c1ccccc1 |
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H311-H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C:Corrosive |
| Risk Phrases | R21/22;R35 |
| Safety Phrases | S26-S28-S36/37/39-S45 |
| RIDADR | UN 2735 |
| WGK Germany | 1 |
| RTECS | DP5775000 |
| Packaging Group | III |
| HS Code | 29214980 |
| Precursor 4 | |
|---|---|
| DownStream 10 | |
| HS Code | 2921499090 |
|---|---|
| Summary | 2921499090 other aromatic monoamines and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Interactions of an asymmetric amine with a non-C2 symmetric Cu-salen complex: an EPR/ENDOR and HYSCORE investigation.
Phys. Chem. Chem. Phys. 13(45) , 20427-34, (2011) Single enantiomers of R-/S-methylbenzylamine (MBA) were found to selectively form adducts with the chiral non-C(2) symmetric Cu-salen complex N-(3,5-di-tert-butylsalicylidene)-N'-(salicylidene)-cycloh... |
|
|
Synthesis, Crystal Structure, Absolute Configuration and Antitumor Activity of the Enantiomers of 5-Bromo-2-chloro-N-(1-phenylethyl)pyridine-3-sulfonamide.
Molecules 20 , 20926-38, (2015) Pyridinesulfonamide is an important fragment which has a wide range of applications in novel drugs. R- and S-isomers of 5-bromo-2-chloro-N-(1-phenylethyl)pyridine-3-sulfonamide have been synthesized, ... |
|
|
Enantioselective nanofiber-spinning of chiral calixarene receptor with guest.
Chem. Commun. (Camb.) (32) , 3398-400, (2007) Chiral para-tert-butylcalix[4]arene bearing (S)-alpha-methylbenzylamine groups at lower rim only self-assembles with one of two enantiomers of 2,3-dibenzoyltartaric acid into coiled nanofibers and the... |
|
Name: Inhibitory activity against bovine adrenal phenylethanolamine N-methyl-transferase (P...
Source: ChEMBL
Target: Phenylethanolamine N-methyltransferase
External Id: CHEMBL760054
|
|
Name: Inhibition of [3H]clonidine binding to the rat alpha-2-adrenoceptor
Source: ChEMBL
Target: Alpha-2A adrenergic receptor
External Id: CHEMBL641405
|
|
Name: Inhibitory activity against Monoamine Oxidase B of Bovine liver in competitive inhibi...
Source: ChEMBL
Target: Amine oxidase [flavin-containing] B
External Id: CHEMBL729340
|
|
Name: Ratio of Ki for rat brain NMDA receptor in presence of 100 uM spermine to Ki for rat ...
Source: ChEMBL
Target: Glutamate receptor ionotropic, NMDA 3A
External Id: CHEMBL978911
|
|
Name: Inhibitory activity against Norepinephrine N-methyl-transferase of bovine adrenal gla...
Source: ChEMBL
Target: Phenylethanolamine N-methyltransferase
External Id: CHEMBL752161
|
|
Name: Inhibitory constant against bovine phenylethanolamine N-methyl-transferase
Source: ChEMBL
Target: Phenylethanolamine N-methyltransferase
External Id: CHEMBL759583
|
|
Name: Displacement of [3H]MK801 from NMDA receptor in rat brain neuronal membrane
Source: ChEMBL
Target: Glutamate receptor ionotropic, NMDA 3A
External Id: CHEMBL978910
|
|
Name: Inhibitory constant against human phenylethanolamine N-methyl-transferase over-expres...
Source: ChEMBL
Target: Phenylethanolamine N-methyltransferase
External Id: CHEMBL759752
|
|
Name: Inhibitory activity against bovine adrenal norepinephrine N-methyl-transferase was de...
Source: ChEMBL
Target: Phenylethanolamine N-methyltransferase
External Id: CHEMBL752162
|
|
Name: Ki ratio of human versus bovine phenylethanolamine N-methyl-transferase
Source: ChEMBL
Target: N/A
External Id: CHEMBL759769
|
| (S)-(−)-1-Phenylethylamine |
| (S)-(-)-α-Methylbenzylamine |
| (1R)-1-Phenylethanamine |
| (s)-benzenemethanamin |
| L-PHENYLETHYLAMINE |
| (S)-1-phenyl-ethylamine |
| N-Ethyl-N-phenylamine |
| L-Phenethylamine |
| (R)-α-Methylbenzenemethanamine |
| (1R)-1-Phenylethylamine |
| (S)-(−)-α-Methylbenzylamine |
| S(-)PHENYLETHYLAMINE |
| N-Ethylaniline |
| ANILINE,N-ETHYL |
| R-(+)-α-Phenylethylamine |
| N-Ethylbenzenamine |
| L(-)-alpha-Methylbenzylamine |
| (1S)-1-PHENYLETHYLAMINE |
| EINECS 220-098-0 |
| 1-Phenylethylamine |
| N-Ethylaniline [UN2272] [Poison] |
| (S)-1-PHENYLETHANAMINE |
| S-(-)-α-phenylethylamine |
| (S)-(-)-1-Phenylethylamine |
| (1R)-(+)-1-Phenylethylamine |
| N-ethylbenzeneamine |
| p-Ethylaminobenzene |
| N-Ethylaminobenzene |
| (-)-PEA |
| (S)-(-)-Alpha-Methylbenzylamine |
| (R)-(+)-α-Methylbenzylamine |
| (R)-1-phenylethylamine |
| (R)-1-phenylethanamine |
| (+)-α-PHENYLETHYLAMINE |
| MFCD00064406 |
| (+)-α-Methylbenzylamine |
| n-Ethyl aniline |
| (S)-(-)-1-METHYLBENZYLAMINE |
| L-1-Phenylethylamine |
![N-[tert-butyl-(S)-sulfinyl]-methylphenylketimine Structure](https://image.chemsrc.com/caspic/413/874291-45-9.png) CAS#:874291-45-9
CAS#:874291-45-9 CAS#:98-86-2
CAS#:98-86-2![N-[(1S)-1-phenylethan-1-yl]-4-methylbenzenesulfonamide Structure](https://image.chemsrc.com/caspic/493/66558-04-1.png) CAS#:66558-04-1
CAS#:66558-04-1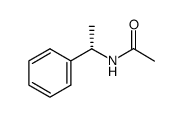 CAS#:19144-86-6
CAS#:19144-86-6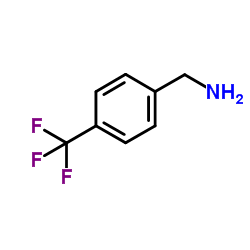 CAS#:3300-51-4
CAS#:3300-51-4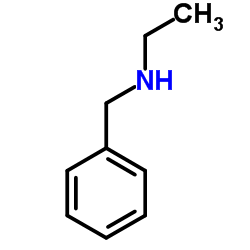 CAS#:3789-59-1
CAS#:3789-59-1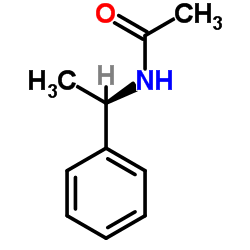 CAS#:36283-44-0
CAS#:36283-44-0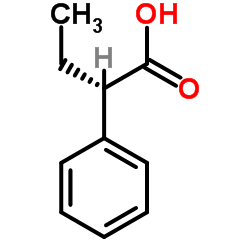 CAS#:4286-15-1
CAS#:4286-15-1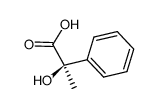 CAS#:3966-30-1
CAS#:3966-30-1 CAS#:106336-16-7
CAS#:106336-16-7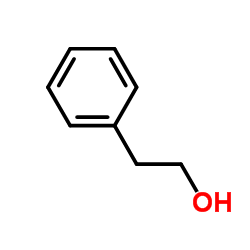 CAS#:98-85-1
CAS#:98-85-1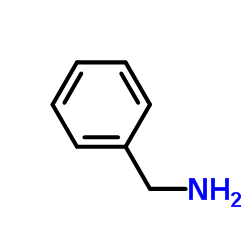 CAS#:100-46-9
CAS#:100-46-9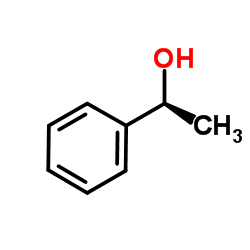 CAS#:1445-91-6
CAS#:1445-91-6
