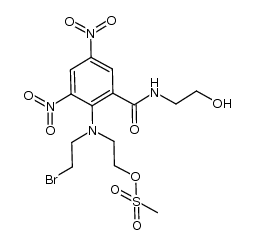
680199-06-8
| Name | 2-((2-bromoethyl)-2-{[(2-hydroxyethyl)-amino]-carbonyl}-4,6-dinitroanilino)-ethyl methanesulfonate |
|---|---|
| Synonyms |
2-((2-BROMOETHYL)-2-{[(2-HYDROXYETHYL)amino]CARBONYL}-4,6-dinitroanilino) ethyl methanesulfonate
2-((2-bromoethyl)-2-{[(2-hydroxyethyl) amino] carbonyl}-4,6-dinitroanilino)ethyl methanesulfonate 2-[(2-bromoethyl)-2-[[(2-hydroxyethyl)amino]carbonyl]-4,6-dinitroanilino]ethyl methanesulfonate 2-((2-bromoethyl)-2-{[(2-hydroxyethyl)amino]carbonyl}-4,6-dinitroanilino)ethyl methanesulfonate |
| Description | PR-104A (SN 27858) is the alcohol metabolite of phosphate prodrug PR-104. PR-104A is a hypoxia-selective DNA cross-linking agent/DNA-damaging agent and cytotoxin. Antitumor Activity[1]. PR-104A is metabolized under hypoxia by the 1-electron NADPH:cytochrome P450 oxidoreductase. PR-104A can be used for the research of relapsed/refractory T-lineage acute lymphoblastic leukemia (T-ALL)[2]. |
|---|---|
| Related Catalog | |
| In Vitro | PR-104A (1-100 uM) shows antiproliferative potency in a panel of 10 human carcinoma cell lines following 4 hours exposures under aerobic and hypoxic conditions with the lowest IC50 (0.51 μM) in H460 non–small cell lung cancer cells and highest (7.3 μM) in PC3 prostate cells[1]. Cell Proliferation Assay[1] Cell Line: HT29 , HCT116, C33A SiHa A549, H460, H1299 ,PC3,SKOV3, A375 cells Concentration: 0, 1, 10, 100 uM Incubation Time: 4 hours under aerobic or hypoxic conditions Result: The lowest IC50 (0.51 μM) in H460 non-small cell lung cancer cells and highest (7.3 μM) in PC3 prostate cells. |
| In Vivo | The phosphate ester “pre-prodrug” PR-104 is well tolerated in mice and converted rapidly to the corresponding prodrug PR-104A. H460 xenografts shows significant sensitivity to PR-104 (total dose 3.2 mmol/kg)[1]. Animal Model: Specific pathogen-free homozygous nude (CD1-Foxn1nu) mice with H460 xenografts[1] Dosage: Daily (0.23 mmol/kg/dose; qd ×14) or weekly (1.07 mmol/kg/dose; qw ×3) Administration: I.p. Result: The single-agent activity against H460 tumors refractory to docetaxel, cisplatin, gemcitabine, and cyclophosphamide was particularly striking. Compared a daily (qd ×14) versus weekly (qw ×3) schedule against the chemoresistant H460 xenograft model using the same total dose (3.2 mmol/kg) over 14 days, which was well tolerated using both schedules. |
| References |
| Molecular Formula | C14H19BrN4O9S |
|---|---|
| Molecular Weight | 499.29100 |
| Exact Mass | 498.00600 |
| PSA | 195.96000 |
| LogP | 2.92070 |