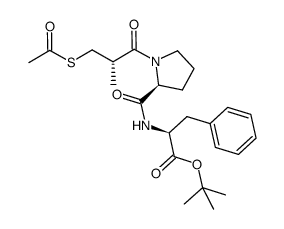
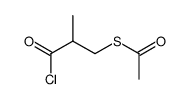
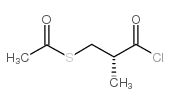
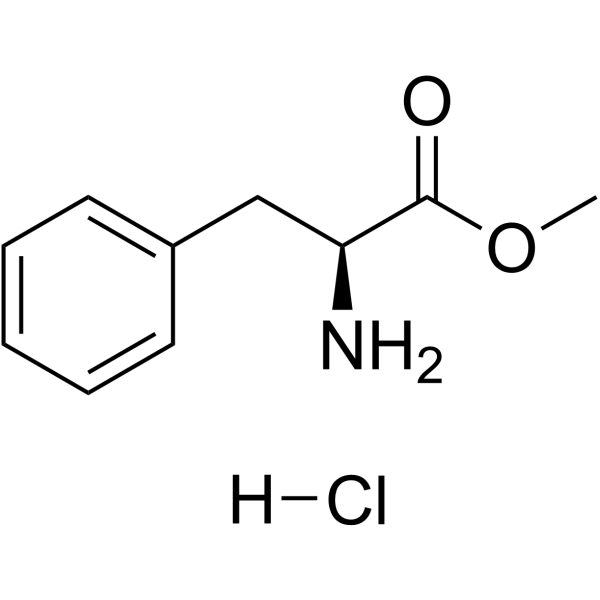
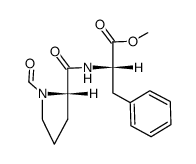
74258-86-9
| Name | Alacepril |
|---|---|
| Synonyms |
1-(d-3-acetylthio-2-methylpropanoyl)-l-prolyl-l-phenylalanine
(S)-N-[1-[3-(acetylthio)-2-methyl-1-oxopropyl]-L-prolyl]-L-phenylalanil L-Phenylalanine,1-[(2S)-3-(acetylthio)-2-methyl-1-oxopropyl]-L-prolyl 1-((S)-3-acetylthio-2-methylpropanoyl)-L-prolyl-L-phenylalanine cetapril 1-[(2S)-3-(acetylthio)-2-methyl-1-oxopropyl]-L-prolyl-L-phenylalanine N-[(S)-3-Acetylthio-2-methylpropanoyl]-L-Pro-L-Phe-OH du1219 Cetapfil (s)-l-phenylalanin 1-[(S)-3-Acetylthio-2-methylpropanoyl]-L-Pro-L-Phe-OH MFCD00869538 |
| Description | Alacepril (Cetapril) is an orally active angiotensin converting enzyme (ACE) inhibitor with long lasting antihypertensive effect[1]. |
|---|---|
| Related Catalog | |
| In Vivo | The long lasting antihypertensive effect of Alacepril in renal hypertensive rats is confirmed by once daily successive oral administration (1-2 mg/kg/d). In renal hypertensive dogs, Alacepril (3 mg/kg) showed a stable and sustained hypotensive effect, and its duration of action was longer than that of captopril[1]. |
| References |
| Density | 1.281g/cm3 |
|---|---|
| Boiling Point | 679.1ºC at 760 mmHg |
| Molecular Formula | C20H26N2O5S |
| Molecular Weight | 406.49600 |
| Flash Point | 364.5ºC |
| Exact Mass | 406.15600 |
| PSA | 129.08000 |
| LogP | 2.03410 |
| Index of Refraction | 1.581 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|---|
| Risk Phrases | R22:Harmful if swallowed. |
| WGK Germany | 3 |
| RTECS | UO3516500 |
|
~99% 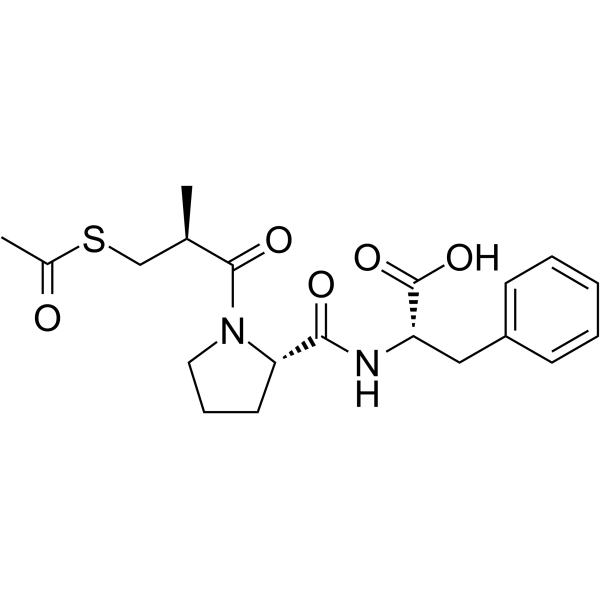
74258-86-9 |
| Literature: NICOX S.A. Patent: WO2004/106300 A1, 2004 ; Location in patent: Page 65 ; |
|
~% 
74258-86-9 |
| Literature: US4248883 A1, ; |
|
~65% 
74258-86-9 |
| Literature: Sawayama; Itokawa; Shimada; Doi; Kimura; Nishim ura Chemical and Pharmaceutical Bulletin, 1990 , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
|
~% 
74258-86-9 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 38, # 2 p. 529 - 531 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |