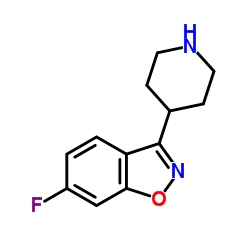
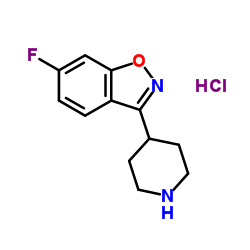
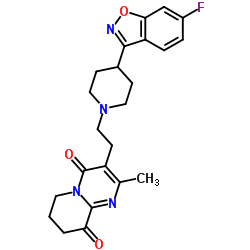
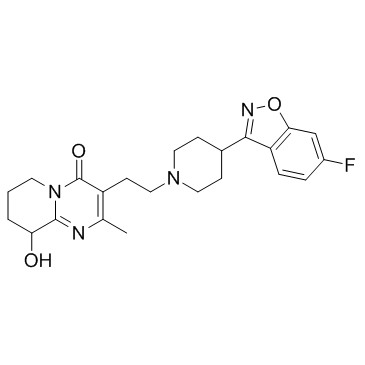
144598-75-4
| Name | Paliperidone |
|---|---|
| Synonyms |
UNII:838F01T721
9-OH-risperidone 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one 9-Hydroxyrisperidone 3-{2-[4-(6-Fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one Paliperidone MFCD00871802 3-{2-[4-(6-Fluoro-1,2-benzoxazol-3-yl)-1-piperidinyl]ethyl}-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one |
| Description | Paliperidone (9-hydroxyrisperidone) is a dopamine antagonist of the atypical antipsychotic class of medications. IC50 value:Target: dopamine receptorin vitro: Paliperidone inhibited MK-801 induced neurotoxicity both in MTT metabolism assay (p<0.01) and in lactate dehydrogenase (LDH) activity assay (p<0.01). Moreover, paliperidone could significantly retard MK-801-mediated inhibition of neurite outgrowth (p<0.01) and reverse MK-801-induced decreases of gene expression and phosphorylation of Akt1 and GSK3β (both p<0.01). Furthermore, these protective effects of paliperidone were blocked by pretreatment with a PI3K inhibitor LY294002 [1]. paliperidone works finely at low concentrations (10 and 50 μM) against Aβ(25-35) and MPP(+) and solely protected SH-SY5Y from hydrogen peroxide. At 100 μM, paliperidone completely diminished cell reduction induced by different stressors, regardless of their dosages. Paliperidone was demonstrated with a higher oxidative stress-scavenging properties than other APDs in several aspects, such as generated bulk glutathione, low HNE, and protein carbonyl productions [2].in vivo: The 9OHRIS (4 mg/bwkg) was administred by gastric tube. Four groups were formed depending on the treatment: (1) control, (2) stress, (3) 9OHRIS, (4) stress and parallel 9OHRIS treatment (n=5-6). The expression of APP, MAPK1, β-actin mRNAs from the perfused brain samples was measured with real-time PCR technique [3].Male offspring were treated orally via drinking water with vehicle, risperidone (0.01mg/kg/day), or paliperidone (0.01mg/kg/day) between postnatal days 35 and 56 (periadolescence) and extracellular glutamate levels in the prefrontal cortex were determined by microdialysis at PD 56 [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 612.3±65.0 °C at 760 mmHg |
| Melting Point | 158-160°C |
| Molecular Formula | C23H27FN4O3 |
| Molecular Weight | 426.484 |
| Flash Point | 324.1±34.3 °C |
| Exact Mass | 426.206726 |
| PSA | 84.39000 |
| LogP | 1.52 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.692 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| RTECS | UV1164720 |
| Precursor 7 | |
|---|---|
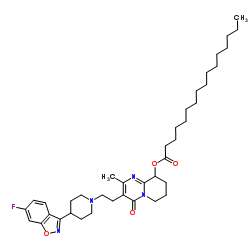
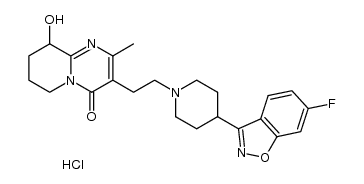
| DownStream 2 | |


![3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-9-(hydroxyimino)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one structure](https://image.chemsrc.com/caspic/014/1204248-69-0.png)
![3-(2-Chloroethyl)-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one structure](https://image.chemsrc.com/caspic/315/130049-82-0.png)