71116-82-0
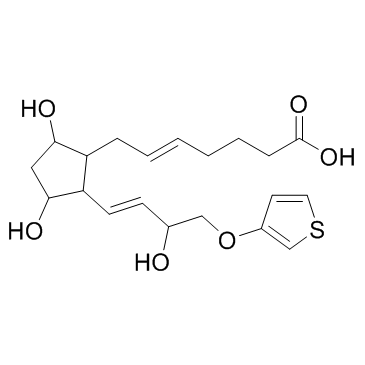
| Name | (E)-7-[3,5-dihydroxy-2-[(E)-3-hydroxy-4-thiophen-3-yloxybut-1-enyl]cyclopentyl]hept-5-enoic acid |
|---|---|
| Synonyms |
Iliren
(5Z)-7-{(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E)-3-hydroxy-4-(3-thienyloxy)-1-buten-1-yl]cyclopentyl}-5-heptenoic acid Tiaprost UNII-98E50HHH7M (15-R,S)-16-(3-Thienyloxy)-w-tetranor-PGF2a (5Z)-7-{(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E)-3-hydroxy-4-(3-thienyloxy)but-1-en-1-yl]cyclopentyl}hept-5-enoic acid Tiaprostum 5-Heptenoic acid,7-(3,5-dihydroxy-2-(3-hydroxy-4-(3-thienyloxy)-1-butenyl)cyclopentyl) Tiaprostum [INN-Latin] |
| Description | Tiaprost is a prostaglandin F2α (PGF2α) analogue. |
|---|---|
| Related Catalog | |
| Target |
PGF2α |
| In Vivo | Plasma progesterone levels decrease sharply within 12 hours after the initial treatment with Tiaprost and within 24 hours reach levels at about 3.18 nM (1 ng/mL). Over the following days, progesterone levels remain either slightly above (cows 1, 3, 5 and 6) or below (cow 2) 3.18 nM. In cow 4, levels remain for 17 days above 3.18 nM and drop below this level thereafter. At term, all cows show the lowest recorded progesterone levels (1 to 2 nM). Repeated luteolytic treatments with Tiaprost (cows 1 and 2) or estradio1 benzoate (cow 3) has no further influence on individual progesterone levels. Treatment with progesterone-releasing intravaginal device (PRID) does not significantly elevate progesterone plasma levels. Total estrogens in plasma remained in general unchanged[1]. |
| Animal Admin | Cows[1] Six healthy dairy cows of the German Black Pied breed, pregnant for 190 to 266 days, are treated initially with 0.75 mg of the PGF2α analog Tiaprost SC (treatment day=day 0). All animals are moved to the department’s large animal hospital 5 to 20 days before treatment commenced; they are kept in stanchions and fed. All animals are examined clinically daily and rectally as well as vaginally at two- to six-day intervals[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 613.4±55.0 °C at 760 mmHg |
| Molecular Formula | C20H28O6S |
| Molecular Weight | 396.498 |
| Flash Point | 324.8±31.5 °C |
| Exact Mass | 396.160645 |
| PSA | 135.46000 |
| LogP | 1.16 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.633 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | UN 3249 |
|---|---|
| Packaging Group | III |
| Hazard Class | 6.1(b) |