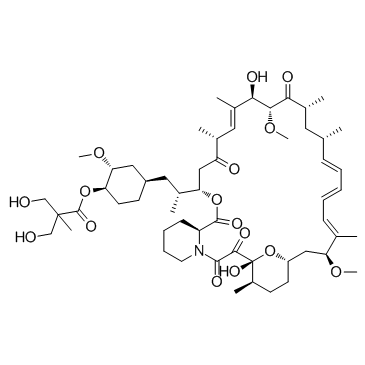
162635-04-3
| Name | Temsirolimus |
|---|---|
| Synonyms |
CCL-779
TeMsiroliMus (~) Torisel Temsirolimus [14C]-Temsirolimus 42-[3-Hydroxy-2-(hydroxymethyl)-2-methylpropanoate]rapamycin Propanoic acid, 3-hydroxy-2-(hydroxymethyl)-2-methyl-, (1R,2R,4S)-2-methoxy-4-[(2R)-2-[(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,26R,27R,34aS)-1,4,5,6,9,10,11,12,13,14,21,22,23,24,25,26,27,28,29,31,32,33,34,34a-tetracosahydro-9,27-dihydroxy-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-1,5,11,28,29-pentaoxo-23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontin-3-yl]propyl]cyclohexyl ester Rapamycin (42-[3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate] TeMsiroliMus (Torisel) Rapamycin 42-[3-Hydroxy-2-(hydroxymethyl)-2-methylpropanoate rapaMycin 42-ester with 3-hydroxy-2-(hydroxyMethyl)-2-Methylpropionic acid (1R,2R,4S)-4-{(2R)-2-[(3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,27-dihydroxy-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-1,5,11,28,29-pentaoxo-1,4,5,6,9,10,11,12,13,14,21,22,23,24,25,26,27,28,29,31,32,33,34,34a-tetracosahydro-3H-23,27-epoxypyrido[2,1-c][1,4]oxazacyclohentriacontin-3-yl]propyl}-2-methoxycyclohexyl 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate (1R,2R,4S)-4-{(2R)-2-[(1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,35R)-1,18-Dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0] hexatriaconta-16,24,26,28-tetraen-12-yl]propyl}-2-methoxycyclohexyl 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate NSC 683864 |
| Description | Temsirolimus is an inhibitor of mTOR with an IC50 of 1.76 μM. |
|---|---|
| Related Catalog | |
| Target |
mTOR:1.76 μM (IC50) |
| In Vitro | Temsirolimus potently inhibits mTOR kinase activity with IC50 of 1.76 μM, similar to that of rapamycin with IC50 of 1.74 μM in the absence of FKBP12. Temsirolimus (10 nM to <5 μM) displays a modest and selective antiproliferative activity via FKBP12-dependent mechanism, but can completely inhibit the proliferation of a broad panel of tumor cells at low micromolar concentrations (5-15 μM), involving FKBP12-independent suppression of mTOR signaling. Temsirolimus treatment at micromolar but not nanomolar concentrations (20 μM) causes a marked decline in global protein synthesis and disassembly of polyribosomes, accompanied by rapid increase in the phosphorylation of translation elongation factor eEF2 and the translation initiation factor eIF2A[1]. Temsirolimus inhibits the phosphorylation of ribosomal protein S6, more potently in PTEN-positive DU145 cells than in PTEN-negative PC-3 cells, and inhibits cell growth and clonogenic survival of both cells in a concentration-dependent manner[2]. Temsirolimus (100 ng/mL) potently inhibits proliferation and induces apoptosis in primary human lymphoblastic leukemia (ALL) cells[3]. |
| In Vivo | CCI-779 (20 mg/kg, i.p.) inhibits the growth of both prostate cancer xenografts, and the rowth of PC-3 tumors is inhibited in a dose-dependent manner and growth inhibition is greater than for DU145 tumors[2]. In the NOD/SCID xenograft models with human ALL, Temsirolimus treatment at 10 mg/kg/day produces a decrease in peripheral blood blasts and in splenomegaly[3]. Administration of Temsirolimus (20 mg/kg, i.p. 5 days/week) significantly delays the growth of DAOY xenografts by 160% after 1 week and 240% after 2 weeks, compared with controls. Single high-dose of Temsirolimus (100 mg/kg, i.p) treatment induces 37% regression of tumor volume within 1 week. Temsirolimus treatment for 2 weeks also delays the growth of rapamycin-resistant U251 xenografts by 148%[4]. Inhibition of mTOR by Temsirolimus improves performance on four different behavioral tasks and decreases aggregate formation in a mouse model of Huntington disease[5]. Administration of Temsirolimus induces significant dose-dependent, antitumor responses against subcutaneous growth of 8226, OPM-2, and U266 xenografts with ED50 of 20 mg/kg and 2 mg/kg for 8226 and OPM-2, respectively, which are associated with inhibited proliferation and angiogenesis, induction of apoptosis, and reduction in tumor cell size[6]. |
| Kinase Assay | The Flag-tagged wild-type human mTOR (Flag-mTOR) DNA constructs are transiently transfected into HEK293 cells. Protein extraction and purification of Flag-mTOR are carried out 48 hours later. In vitro kinase assays of purified Flag-mTOR in the presence of various concentrations of Temsirolimus without FKBP12 are performed in 96-well plate and detected by dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA) using His6-S6K1 as the substrate. Enzymes is first diluted in kinase assay buffer (10 mM Hepes (pH 7.4), 50 mM NaCl, 50 mM β-glycerophosphate, 10 mM MnCl2, 0.5 mM DTT, 0.25 μM microcystin LR, and 100 μg/mL BSA). To each well, 12 μL of the diluted enzyme is mixed briefly with 0.5 μL Temsirolimus. The kinase reaction is initiated by adding 12.5 μL kinase assay buffer containing ATP and His6-S6K to give a final reaction volume of 25 μL containing 800 ng/mL FLAG-mTOR, 100 μM ATP, and 1.25 μM His6-S6K. The reaction plate is incubated for 2 hours (linear at 1-6 hours) at room temperature with gentle shaking and then terminated by adding 25 μL Stop buffer (20 mM Hepes (pH 7.4), 20 mM EDTA, and 20 mM EGTA). The DELFIA detection of the phosphorylated (Thr-389) His6-S6K is performed at room temperature using a monoclonal anti-P(T389)-p70S6K antibody labeled with Europium-N1-ITC (Eu) (10.4 Eu per antibody). 45 μL of the terminated kinase reaction mixture is transferred to a MaxiSorp plate containing 55 μL PBS. The His6-S6K is allowed to attach for 2 hours after which the wells are aspirated and washed once with PBS. 100 μL of DELFIA buffer with 40 ng/mL Eu-P(T389)-S6K antibody is added. The antibody binding is continued for 1 hour with gentle agitation. The wells are then aspirated and washed four times with PBS containing 0.05% Tween 20 (PBST). 100 μL of DELFIA Enhancement solution is added to each well and the plates are read in a PerkinElmer Victor model plate reader. |
| Cell Assay | Survival of prostate cancer cells following various treatments is also determined in a colony-forming assay. Exponentially growing cells are exposed to varying doses of mitoxantrone or docetaxel for 24 hours, or to CCI-779 for 3 days. Following this treatment, the cells are washed and trypsinized. Serial dilutions are plated in 6-well plates in 5 mL medium. The plates are incubated for 10 days at 37°C in an atmosphere containing 5% CO2 at 90% humidity. The plates are then stained with methylene blue and colonies containing >50 cells are counted. |
| Animal Admin | For generation of xenografts, cells are implanted in matrigel; matrigel is stored at −20°C and then thawed on ice at 4°C for 3 hours before use. Cells are gently resuspended in 1 mL of PBS and incubated on ice for 5 minutes. A prechilled pipette is used to transfer cells to the tube containing 1 mL of matrigel, and the cell concentration is adjusted to 3×107/mL. The cells (3×106 in 0.1 mL) are injected s.c. into both flanks of mice using a 25-gauge needle. When xenografts grew to a size of about 5 mm in diameter, animals are assorted randomLy into groups of 10 mice. The following experiments are conducted: Mice bearing PC-3 tumors are treated with CCI-779 (1, 5, 10, and 20 mg per kg per day), or vehicle solution for 3 or 5 days per week for 3 weeks. Mice bearing DU145 tumors are only treated with CCI-779 (20 mg per kg per day) or vehicle solution for 3 weeks. Mice bearing PC-3 tumors receive the following treatments: (a) control, vehicle solution for CCI-779; (b) chemotherapy alone, mitoxantrone 1.5 mg/kg or docetaxel 10 mg/kg is injected i.p. weekly for 3 doses; (c) CCI-779 alone, 5 or 10 mg/kg is injected i.p. daily, three times a week for 3 weeks; (4) chemotherapy followed by CCI-779. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 1048.4±75.0 °C at 760 mmHg |
| Melting Point | 99-101ºC |
| Molecular Formula | C56H87NO16 |
| Molecular Weight | 1030.287 |
| Flash Point | 587.8±37.1 °C |
| Exact Mass | 1029.602539 |
| PSA | 241.96000 |
| LogP | 2.96 |
| Appearance | white to off-white |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.554 |
| Storage condition | -20°C Freezer |
| Water Solubility | Soluble in chloroform, methanol. |
| Safety Phrases | 24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | VE6257000 |
| HS Code | 29349990 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |