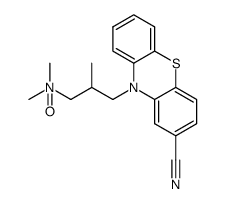
3546-03-0
| Name | Cyamemazine |
|---|---|
| Synonyms |
Kyamepromazin
Kyamepromazine 7204 R.E. Ciamatil Cyamemazine 10-[3-(Dimethylamino)-2-methylpropyl]-10H-phenothiazine-2-carbonitrile 2-Cyano-10-(3-dimethylamino-2-methylpropyl)phenothiazine Tercian 10-[3-(dimethylamino)-2-methylpropyl]phenothiazine-2-carbonitrile |
| Description | Cyamemazine is a neuroleptic agent that contains the phenothiazine chromophore. Cyamemazine is often used as an anxiolytic. Cyamemazine is a potent 5-HT3 (Ki of 12 nM), 5-HT2A (Ki = 1.5 nM) and 5-HT2C (Ki of 75 nM) receptors antagonist with antipsychotic activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A Receptor:1.5 nM (Ki) 5-HT2C Receptor:12 nM (Ki) 5-HT3 Receptor:75 nM (Ki) |
| In Vitro | Cyamemazine exhibits a high affinity for dopamine receptors, which is consistent with its antipsychotic activity. The antagonist activity of Cyamemazine at muscarinic receptors is consistent with its affinity for M1 (Ki = 13 nM), M2 (Ki = 42 nM), M3 (Ki = 321 nM), M4 (Ki = 12 nM), and M5 (Ki = 35 nM) receptors[1]. |
| In Vivo | Cyamemazine behaves as an antagonist at the 5-HT3, 5-HT2C, and 5-HT2A receptors in 5-HT3-dependent contraction of isolated guinea pig ileum and bradycardic responses in rats, in 5-HT2C-dependent phospholipase C stimulation in the rat brain membrane, and in 5-HT2A-dependent contraction of isolated rat aorta rings and isolated guinea pig trachea. Cyamemazine antagonizes 5-HT3 and 5-HT2C receptors and that this effect is partially involved in its therapeutic activity in anxiety disorders. Acute administration of low doses of Cyamemazine can reduces extracellular dopamine and metabolite concentrations in rat striatum[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 479.0±45.0 °C at 760 mmHg |
| Molecular Formula | C19H21N3S |
| Molecular Weight | 323.455 |
| Flash Point | 243.5±28.7 °C |
| Exact Mass | 323.145630 |
| PSA | 55.57000 |
| LogP | 4.62 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.655 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H410 |
| Precautionary Statements | P273-P501 |
| Hazard Codes | Xi |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 2934300000 |
|
~0% 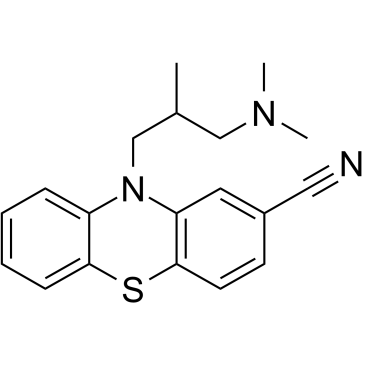
3546-03-0 |
| Literature: Singh, Gurmit; Koerner, Terry B.; Godefroy, Samuel Benrejeb; Armand, Claude Bioorganic and Medicinal Chemistry Letters, 2012 , vol. 22, # 6 p. 2160 - 2162 |
|
~% 
3546-03-0 |
| Literature: Journal of Organic Chemistry, , vol. 26, p. 1138 - 1143 |
|
~% 
3546-03-0 |
| Literature: Journal of Organic Chemistry, , vol. 26, p. 1138 - 1143 |
|
~% 
3546-03-0 |
| Literature: US2877224 , ; |
|
~% 
3546-03-0 |
| Literature: US2877224 , ; |
|
~% 
3546-03-0 |
| Literature: Journal of Organic Chemistry, , vol. 26, p. 1138 - 1143 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934300000 |
|---|---|
| Summary | 2934300000. other compounds containing in the structure a phenothiazine ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |