18642-23-4
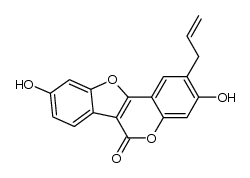
| Name | psoralidin |
|---|---|
| Synonyms |
Psoralidin
3,9-dihydroxy-2-(3-methylbut-2-enyl)-[1]benzofuro[3,2-c]chromen-6-one 3,9-Dihydroxy-2-prenylcoumestan 3,9-Dihydroxy-2-(3-methyl-2-buten-1-yl)-6H-[1]benzofuro[3,2-c]chromen-6-one 3,9-Dihydroxy-2-(3-methyl-2-butenyl)-6H-benzofuro[3,2-c][1]benzopyran-6-one 3,9-Dihydroxy-2-(3-methyl-2-butenyl)-6H-benzofuro(3,2-c)(1)benzopyran-6-one 6-(3-Methylbut-2-enyl)coumestrol 3,9-Dihydroxy-2-(3-methylbut-2-en-1-yl)-6H-[1]benzofuro[3,2-c]chromen-6-one |
| Description | Psoralidin, a natural furanocoumarin, is isolated from Psoralea corylifolia L. possessing anti-cancer properties.IC50 value:Target: Anticancer natural compoundin vitro: PSO dramatically decreased the cell viabilities in dose- and time-dependent manner. Autophagy inhibitor 3-MA blocked the production of LC3-II and reduced the cytotoxicity in response to PSO. Furthermore, PSO increased intracellular ROS level which was correlated to the elevation of LC3-II [1]. Psoralidin at 10 μM was able to induce the maximum reporter gene expression corresponding to that of E2-treated cells and such activation of the ERE-reporter gene by psoralidin was completely abolished by the cotreatment of a pure ER antagonist, implying that the biological activities of psoralidin are mediated by ER [2]. Psoralidin enhanced TRAIL-induced apoptosis in HeLa cells through increased expression of TRAIL-R2 death receptor and depolarization of mitochondrial membrane potential [3]. Psoralidin inhibited the IR-induced COX-2 expression and PGE(2) production through regulation of PI3K/Akt and NF-κB pathway. Also, psoralidin blocked IR-induced LTB(4) production, and it was due to direct interaction of psoralidin and 5-lipoxygenase activating protein (FLAP) in 5-LOX pathway. IR-induced fibroblast migration was notably attenuated in the presence of psoralidin [4].in vivo: Moreover, in vivo results from mouse lung indicate that psoralidin suppresses IR-induced expression of pro-inflammatory cytokines (TNF-α, TGF-β, IL-6 and IL-1 α/β) and ICAM-1[4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 458.8±34.0 °C at 760 mmHg |
| Melting Point | 290-292° |
| Molecular Formula | C20H16O5 |
| Molecular Weight | 336.338 |
| Flash Point | 231.3±25.7 °C |
| Exact Mass | 336.099762 |
| PSA | 83.81000 |
| LogP | 5.03 |
| Appearance | white to beige |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.689 |
| Storage condition | ?20°C |
| Water Solubility | DMSO: soluble5mg/mL, clear (warmed) |
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2932999099 |
|
~70% 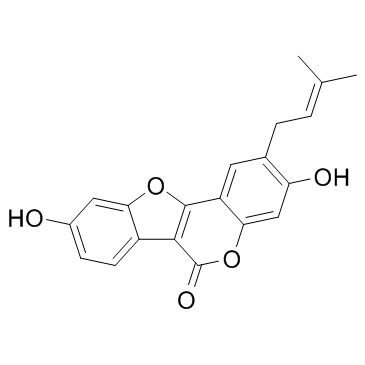
18642-23-4 |
| Literature: Journal of Organic Chemistry, , vol. 74, # 7 p. 2750 - 2754 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |