17598-65-1
| Name | deslanoside |
|---|---|
| Synonyms |
Deacetyllanatoside C
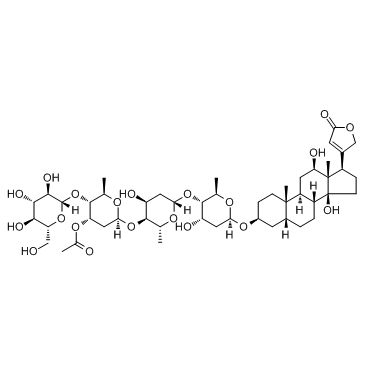
Desacetyllanatoside C Deslanatoside C Deslanoside Deacetyllanatoside C,Desacetyllanatoside C Cedilanide (3β,5β,12β)-3-{[β-D-Glucopyranosyl-(1->4)-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-β-D-ribo-hexopyranosyl]oxy}-12,14-dihydroxycard-20(22)-enolide deacetyl-lanatoside 4-18-00-02455 (Beilstein Handbook Reference) Desacetyldigilanide C Descetyldigilanide C DESLANOSIDUM 4-[(3S,5R,8R,9S,10S,12R,13S,14S,17R)-12,14-Dihydroxy-3-{[(2R,4S,5S,6R)-4-hydroxy-5-{[(2S,4S,5S,6R)-4-hydroxy-5-{[(2S,4S,5S,6R)-4-hydroxy-6-methyl-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-2-yl]oxy}-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6-methyltetrahydro-2H-pyran-2-yl]oxy}-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2(5H)-furanone Desaci Desace MFCD00135818 deacetyl-lanatoside C Deslanatoside Purpurea Glycoside C Desacetyllanatosid C glucodigoxin Deslanosidum C (3β,5β,12β)-3-{[β-D-Glucopyranosyl-(1->4)-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-β-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-β-D-ribo-hexopyranosyl]oxy}-12,14-dihydro
 xycard-20(22)-enolide 4-[(3S,5R,8R,9S,10S,12R,13S,14S,17R)-12,14-Dihydroxy-3-{[(2R,4S,5S,6R)-4-hydroxy-5-{[(2S,4S,5S,6R)-4-hydroxy-5-{[(2S,4S,5S,6R)-4-hydroxy-6-méthyl-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}tétrahydro-2H-pyran-2-yl]oxy}-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-6-méthyltétrahydro-2H-pyran-2-yl]oxy}-10,13-diméthylhexadécahydro-1H-cyclopenta[a]phénanthrén-17-yl]-2(5H)-furanone cedilanidd sediranido EINECS 241-568-1 de-O-acetyl-lanatoside C 4-[(3S,5R,8R,9S,10S,12R,13S,14S,17R)-12,14-Dihydroxy-3-{[(2R,4S,5S,6R)-4-hydroxy-5-{[(2S,4S,5S,6R)-4-hydroxy-5-{[(2S,4S,5S,6R)-4-hydroxy-6-methyl-5-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}tetrahydro-2H-pyran-2-yl]oxy}-6-methyltetrahydro-2H-pyran-2-yl]oxy}-6-methyltetrahydro-2H-pyran-2-yl]oxy}-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2(5H)-furanon |
| Description | Deslanoside (Desacetyllanatoside C) is a rapidly acting cardiac glycoside used to treat congestive heart failure and supraventricular arrhythmias due to reentry mechanisms, and to control ventricular rate in the treatment of chronic atrial fibrillation. Deslanoside inhibits the Na-K-ATPase membrane pump, resulting in an increase in intracellular sodium and calcium concentrations [1][2][3]. |
|---|---|
| Related Catalog | |
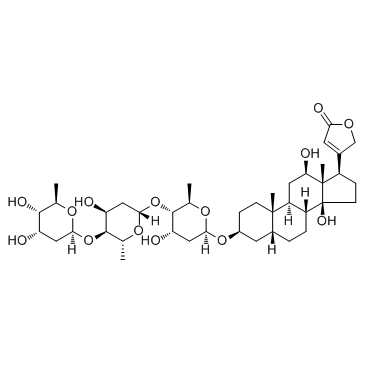
| In Vitro | Deslanoside (Desacetyllanatoside C) is a metabolite of Lanatoside C[4]. Deslanoside increases forearm blood flow and cardiac index and decreased heart rate concomitant with a marked decrease in skeletal muscle sympathetic nerve activity measured as an indicator of centrally mediated sympathetic nervous system activity[1]. |
| References |
[1]. Hauptman PJ, et al. Digitalis. Circulation. 1999 Mar 9;99(9):1265-70. [3]. Deslanoside. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 220-235ºC |
| Molecular Formula | C47H74O19 |
| Molecular Weight | 943.079 |
| Exact Mass | 942.482422 |
| PSA | 282.21000 |
| LogP | -1.40 |
| Index of Refraction | 1.620 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
17598-65-1 |
| Literature: Helvetica Chimica Acta, , vol. 16, p. 1390,1407 Helvetica Chimica Acta, , vol. 17, p. 592,613 |
|
~% 
17598-65-1 |
| Literature: Advanced Synthesis and Catalysis, , vol. 355, # 13 p. 2518 - 2524 |
|
~91% 
17598-65-1 |
| Literature: Muramatsu, Wataru; Yoshimatsu, Hirofumi Advanced Synthesis and Catalysis, 2013 , vol. 355, # 13 p. 2518 - 2524 |
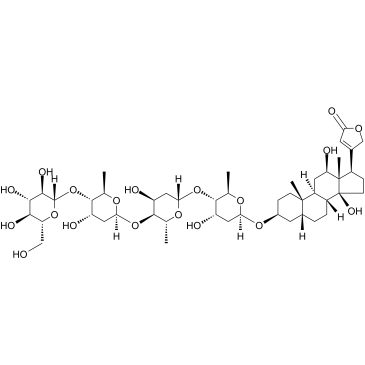
| Precursor 3 | |
|---|---|
| DownStream 1 | |