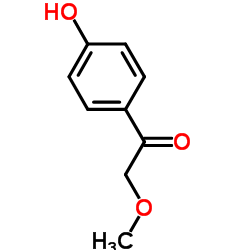
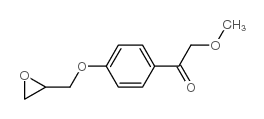
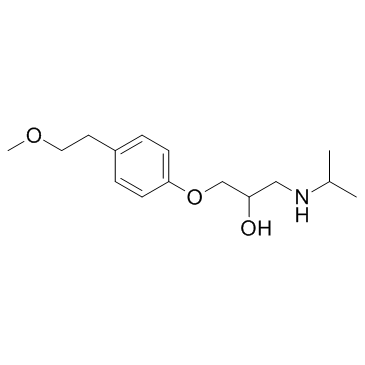
56392-16-6
| Name | α-Hydroxy Metoprolol |
|---|---|
| Synonyms |
1-[4-(1-Hydroxy-2-methoxyethyl)phenoxy]-3-(isopropylamino)-2-propanol
3-[4-(1-Hydroxy-2-methoxyethyl)phenoxy]-1-isopropylamino-2-propanol α-HYDROXYMETOPROLOL 1-[4-(1-hydroxy-2-methoxyethyl)phenoxy]-3-(isopropylamino)propan-2-ol UNII:C19D0413EL |
| Description | a-Hydroxymetoprolol is a metabolite of metoprolol. The adrenoreceptor blocking effect of a-Hydroxymetoprolol on metoprolol is almost zero[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 437.6±45.0 °C at 760 mmHg |
| Melting Point | 65-67ºC |
| Molecular Formula | C15H25NO4 |
| Molecular Weight | 283.363 |
| Flash Point | 218.4±28.7 °C |
| Exact Mass | 283.178345 |
| PSA | 70.95000 |
| LogP | 0.72 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.524 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|
|
~95% 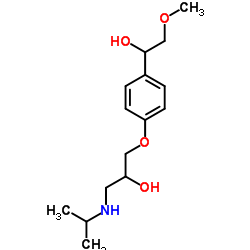
56392-16-6 |
| Literature: Shetty, H. Umesha; Nelson, Wendel L. Journal of Medicinal Chemistry, 1988 , vol. 31, # 1 p. 55 - 59 |
|
~% 
56392-16-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 55 - 59 |
|
~% 
56392-16-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 55 - 59 |
|
~% 
56392-16-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 55 - 59 |
|
~% 
56392-16-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 55 - 59 |
|
~% 
56392-16-6 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 55 - 59 |
|
~% 
56392-16-6 |
| Literature: European Journal of Clinical Pharmacology, , vol. 59, # 5-6 p. 429 - 442 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |