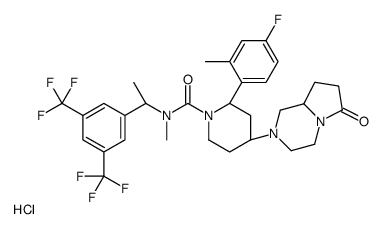
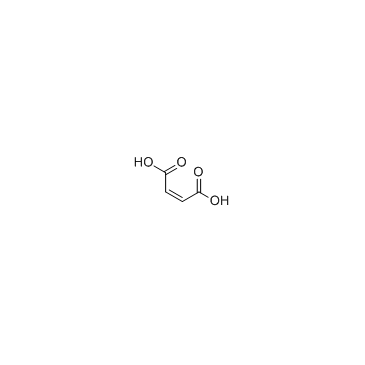
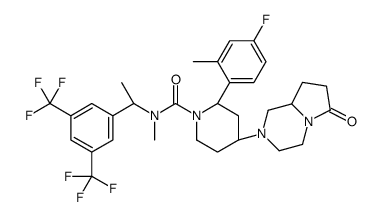
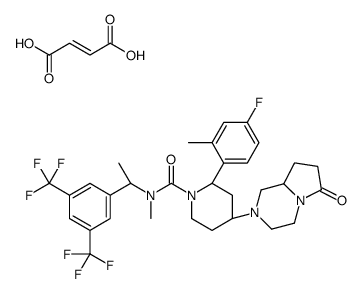
579475-24-4
| Name | (2R,4S)-4-[(8aS)-6-oxo-1,3,4,7,8,8a-hexahydropyrrolo[1,2-a]pyrazin-2-yl]-N-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethyl]-2-(4-fluoro-2-methylphenyl)-N-methylpiperidine-1-carboxamide,(Z)-but-2-enedioic acid |
|---|---|
| Synonyms |
(2R,4S)-N-{(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethyl}-2-(4-fluoro-2-methylphenyl)-N-methyl-4-[(8aS)-6-oxohexahydropyrrolo[1,2-a]pyrazin-2(1H)-yl]-1-piperidinecarboxamide maleate
Orvepitant maleate (USAN) ORVEPITANT MALEATE UNII-HAX0H28B6W |
| Description | Orvepitant maleate (GW823296 maleate) is potent, selective, orally active and well-tolerated neurokinin-1 receptor (NK-1) antagonist with a pKi of 10.2 for human neurokinin-1 receptor. Orvepitant maleate can across the blood-brain barrier. Orvepitant maleate has the potential for depressive disorder and chronic refractory cough (CRC) treatment[1][2]. |
|---|---|
| Related Catalog | |
| Target |
NK1:10.2 (pKi) |
| In Vitro | Orvepitant (Compound 3a) is further characterized in terms of the ability to functionally inhibit substance P (SP)-induced release of cytosolic Ca2+ in human neurokinin-1 receptor (hNK1)-CHO cells. Orvepitant (0.3-10 nM), pre-incubated for 1 h at 37℃ before adding the agonist SP, produces a non-surmountable antagonism of agonist concentration-response curve. For Orvepitant apparents pKB value of 10.30[1]. |
| In Vivo | Orvepitant (Compound 3a; 0.3-10 mg/kg; Oral administration; marmoset) treatment shows a dose dependant reduction of the number of postures was observed at 1 mg/kg (34.9% reduction), 3 mg/kg (36.6% reduction) and 10 mg/kg (46.4% reduction), suggesting a potential anxiolytic-like effect of the compound[1]. Orvepitant (compound 3a) shows an oral bioavailability (F) of 17% in rat and 55% in dog, plasma clearance (Clp) of 29 mL/min/kg in rat and 6 mL/min/kg in dog and a half-life of 2.3 h in rat and 6.1 h in dog. As far as the brain penetration in rats is concerned, a B/P ratio of 1.2 is observed 5 min after the i.v. administration of a 1 mg/kg dose of Orvepitant[1]. Animal Model: Human threat test in the marmoset (HTT)[1] Dosage: 0.3 mg/kg, 1 mg/kg, 3 mg/kg and 10 mg/kg Administration: Oral administration Result: A dose dependant reduction of the number of postures was observed at 1 mg/kg (34.9% reduction), 3 mg/kg (36.6% reduction) and 10 mg/kg (46.4% reduction). |
| References |
| Molecular Formula | C35H39F7N4O6 |
|---|---|
| Molecular Weight | 744.69600 |
| Exact Mass | 744.27600 |
| PSA | 121.70000 |
| LogP | 6.32230 |
|
~% 
579475-24-4 |
| Literature: GLAXO GROUP LIMITED Patent: WO2009/124996 A1, 2009 ; Location in patent: Page/Page column 18 ; |
|
~% 
579475-24-4 |
| Literature: GLAXO GROUP LIMITED Patent: WO2009/124996 A1, 2009 ; Location in patent: Page/Page column 15-17 ; |
|
~% 
579475-24-4 |
| Literature: GLAXO GROUP LIMITED Patent: WO2009/124996 A1, 2009 ; Location in patent: Page/Page column 19-21 ; |