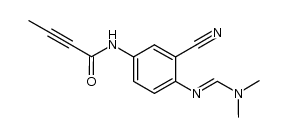
194423-06-8
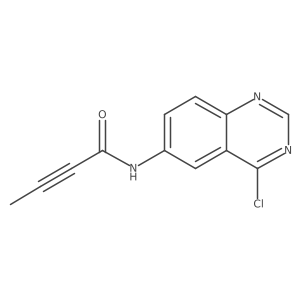
| Name | N-[4-(3-bromoanilino)quinazolin-6-yl]but-2-ynamide |
|---|---|
| Synonyms |
N-{4-[(3-Bromophenyl)amino]-6-quinazolinyl}-2-butynamide
N-{4-[(3-bromophenyl)amino]quinazolin-6-yl}but-2-ynamide CL-387,785 N-[4-[(3-Bromophenyl)amino]quinazolin-6-yl]but-2-ynamide N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-butynamide EKI-785 UNII-B4W27J1Z8B 4-anilinoquinazoline deriv. 1 CL-387785 |
| Description | CL-387785(EKI785; WAY-EKI 785) is an irreversible inhibitor of EGFR with IC50 of 370+/-120 pM; is able to overcome resistance caused by the T790M mutation on a functional level.IC50 value: 370+/-120 pMTarget: EGFRin vitro: CL-387785 blocked EGF-stimulated autophosphorylation of the receptor in cells (ic50 approximately 5 nM), inhibited cell proliferation (IC50 = 31-125 nM) primarily in a cytostatic manner in cell lines that overexpress EGF-R or c-erbB-2 [1]. Whereas transformation by most EGFR mutants confers on cells sensitivity to erlotinib and gefitinib, transformation by an exon 20 insertion makes cells resistant to these inhibitors but more sensitive to the irreversible inhibitor CL-387,785 [3]. CL-387,785 is able to overcome resistance caused by the T790M mutation on a functional level, correlating with effective inhibition of downstream signaling pathways [4].in vivo: CL-387785 profoundly blocked the growth of a tumor that overexpresses EGF-R in nude mice (when given orally at 80 mg/kg/day for 10 days, daily) [1]. Treatment of BPK mice with EKI-785 resulted in a marked reduction of collecting tubule (CT) cystic lesions, improved renal function, decreased biliary epithelial abnormalities, and an increased life span. Cystic animals treated with EKI-785 to postnatal day 48 (P-48) were alive and well with normal renal function, a reduced CT cystic index of 2.0 (P < 0.02), a threefold increased in maximum urinary concentrating ability (P < 0.01), and a significant decrease in biliary epithelial proliferation/fibrosis (P < 0.01) [2]. |
|---|---|
| Related Catalog | |
| Target |
EGFR:370 pM (IC50) |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Melting Point | 276 °C |
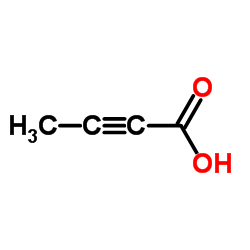
| Molecular Formula | C18H13BrN4O |
| Molecular Weight | 381.226 |
| Exact Mass | 380.027252 |
| PSA | 70.40000 |
| LogP | 4.68 |
| Index of Refraction | 1.755 |
|
~4% 
194423-06-8 |
| Literature: Klutchko, Sylvester R.; Zhou, Hairong; Winters, R. Thomas; Tran, Tuan P.; Bridges, Alexander J.; Althaus, Irene W.; Amato, Danielle M.; Elliott, William L.; Ellis, Paul A.; Meade, Mary Ann; Roberts, Billy J.; Fry, David W.; Gonzales, Andrea J.; Harvey, Patricia J.; Nelson, James M.; Sherwood, Veronica; Han, Hyo-Kyung; Pace, Gerry; Smaill, Jeff B.; Denny, William A.; Showalter, H. D. Hollis Journal of Medicinal Chemistry, 2006 , vol. 49, # 4 p. 1475 - 1485 |
|
~% 
194423-06-8 |
| Literature: Wyeth Holdings Corporation Patent: EP1000039 B1, 2004 ; Location in patent: Page 34 ; |
|
~%
Detail
|
| Literature: American Cyanamid Company Patent: US6323209 B1, 2001 ; |
|
~% 
194423-06-8 |
| Literature: Wyeth Holdings Corporation Patent: EP1000039 B1, 2004 ; Location in patent: Page 35 ; |
|
~% 
194423-06-8 |
| Literature: Tsou; Mamuya; Johnson; Reich; Gruber; Ye; Nilakantan; Shen; Discafani; DeBlanc; Davis; Koehn; Greenberger; Wang; Wissner Journal of Medicinal Chemistry, 2001 , vol. 44, # 17 p. 2719 - 2734 |
|
~% 
194423-06-8 |
| Literature: Tsou; Mamuya; Johnson; Reich; Gruber; Ye; Nilakantan; Shen; Discafani; DeBlanc; Davis; Koehn; Greenberger; Wang; Wissner Journal of Medicinal Chemistry, 2001 , vol. 44, # 17 p. 2719 - 2734 |