136470-78-5
| Name | abacavir |
|---|---|
| Synonyms |
MFCD00903850
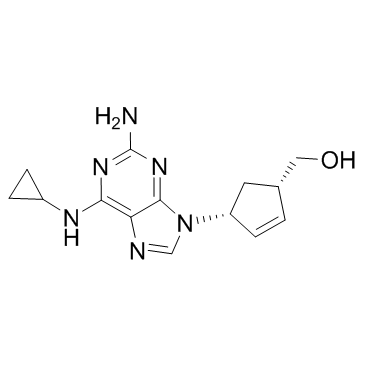
(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol (1S,cis)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol Abacavir {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopenten-1-yl}methanol {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol {(4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol |
| Description | Abacavir is a powerful nucleoside analog reverse transcriptase inhibitor (NRTI) used to treat HIV and AIDS. IC50 value:Target: NRTI; reverse transcriptase inhibitorAbacavir is a nucleoside reverse transcriptase inhibitor marketed since 1999 for the treatment of infection with the human immunodeficiency virus type 1 (HIV). Despite its clinical efficacy, abacavir administration has been associated with serious and sometimes fatal toxic events. Abacavir has been reported to undergo bioactivation in vitro, yielding reactive species that bind covalently to human serum albumin, but the haptenation mechanism and its significance to the toxic events induced by this anti-HIV drug have yet to be elucidated. The mechanism underlying abacavir hypersensitivity syndrome is related to the change in the HLA-B*5701 protein product. Abacavir binds with high specificity to the HLA-B*5701 protein, changing the shape and chemistry of the antigen-binding cleft. This results in a change in immunological tolerance and the subsequent activation of abacavir-specific cytotoxic T cells, which produce a systemic reaction known as abacavir hypersensitivity syndrome. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 636.0±65.0 °C at 760 mmHg |
| Melting Point | 161 °C(dec.) |
| Molecular Formula | C14H18N6O |
| Molecular Weight | 286.332 |
| Flash Point | 338.4±34.3 °C |
| Exact Mass | 286.154205 |
| PSA | 101.88000 |
| LogP | 0.72 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.864 |
| Storage condition | 0-6°C |
| Hazard Codes | T,N |
|---|---|
| Risk Phrases | R21:Harmful in contact with skin. R23/25:Toxic by inhalation and if swallowed . R43:May cause sensitization by skin contact. R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . |
| Safety Phrases | S36/37-S45-S60-S61 |
| RIDADR | UN 2810 |
| RTECS | TE3850000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| Precursor 6 | |
|---|---|
| DownStream 0 | |
![(-)-N-{6-(cyclopropylamino)-9-[(1R,4S)-4-(hydroxymethyl)cyclopent-2-enyl]-9H-purin-2-yl}isobutyramide structure](https://image.chemsrc.com/caspic/130/136470-88-7.png)

![[4S,2R,3R]-(4-benzyl-2-thioxooxazolidin-3-yl)(2-hydroxycyclopent-3-enyl)methanone structure](https://image.chemsrc.com/caspic/062/268737-90-2.png)