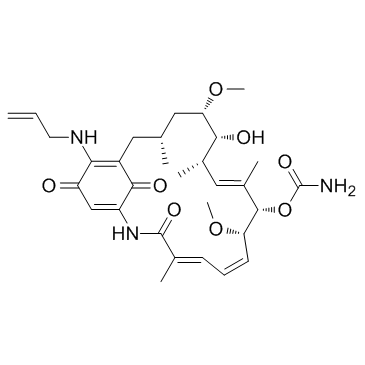
857402-23-4
| Name | 17-allylamino-17-demethoxygeldanamycin hydroquinone |
|---|---|
| Synonyms |
retaspimycin
18,21-DIDEHYDRO-17-DEMETHOXY-18,21-DIDEOXO-18,21-DIHYDROXY-17-(2-PROPENYLAMINO)GELDANAMYCIN (4E,6Z,8S,9S,10E,12S,13R,14S,16R)-19-(Allylamino)-13,20,22-trihydroxy-8,14-dimethoxy-4,10,12,16-tetramethyl-3-oxo-2-azabicyclo[16.3.1]docosa-1(22),4,6,10,18,20-hexaen-9-yl carbamate |
| Description | Retaspimycin is a potent and water-soluble inhibitor of Hsp90, with EC50s of 119 nM for both Hsp90 and Grp9. |
|---|---|
| Related Catalog | |
| Target |
HSP90:119 nM (EC50) GRP94:119 nM (EC50) |
| In Vitro | Retaspimycin is a potent inhibitor of Hsp90, with EC50s of 119 nM for both Hsp90 and Grp9. Retaspimycin (IPI-504) is cytocoxic to human multiple myeloma (MM) cell lines, with EC50s of 307 ± 51 nM and 306 ± 38 nM, respectively, for MM1.s and RPMI-8226 cells[1]. Retaspimycin (IPI-504, 10-100 nM) suppresses the growth of both trastuzumab-sensitive and -resistant cells in a dose-dependent manner. Retaspimycin (0-500 nM) decreases HER2 protein expression and suppresses both Akt and MAPKs pathways in both sensitive and trastuzumab-resistant cells[3]. |
| In Vivo | Retaspimycin (IPI-504, 50 mg/kg, i.v.) causes selective tumor retention in RPMI-8226 tumor-bearing mice[1]. Retaspimycin (IPI-504, 100 mg/kg, p.o., 3 times per week) reduces the tumor volume by 69% and and 84% of baseline values in GIST-882 and GIST-PSW xenografts, respectively. Furthermore, Retaspimycin in combination with imatinib inhibits tumor growth more significantly than Retaspimycin alone in GIST-PSW model, but no obvious difference is ovsrebed in the GIST-882 model. Retaspimycin also downregulates KIT in gastrointestinal stromal tumor (GIST)[2]. Retaspimycin (IPI-504, 50 mg/kg) shows antitumor activity in HCC1569 xenografts. IPI-504 (100 mg/kg, i.p.) effectively decreases the levels of HER2, p-Akt, and p-MAPKs in BT474R and BT474H1047R tumors[3]. |
| Cell Assay | Cell proliferation is studied using the cell proliferation reagent WST-1. Briefly, 8 × 103 cells are seeded in triplicate in 96-well plates and treated for 5 days, with either trastuzumab or Retaspimycin as indicated. Viable cells are estimated on the basis of their ability to metabolize tetrazolium salt WST-1 to formazan by mitochondrial dehydrogenases. Quantification of the formazan dye directly correlates with the number of metabolically active cells and is analyzed by a scanning microplate reader. Results are shown as means ± SE[3]. |
| Animal Admin | RPMI-8226 cells are harvested from cultures grown in vitro in RPMI medium 1640 supplemented with heat-inactivated 10% (wt/vol) FBS and 100 units/mL penicillin/streptomycin at 37°C under a humidified 95%/5% (vol/vol) mixture of air and CO2. Cells are washed twice by using sterile Hepes-buffered saline (HBS) and suspended in HBS to a concentration of 1 × 108 viable cells per mL. Twelve female Nu/Nu nude mice (≈20 g) are used in the assay. RPMI-8226 cells (1 × 107 cells per mouse) are implanted in the right flank. When tumor volume reaches ≈200-500 mm3 (≈4 weeks postimplantation), animals receive a single i.v. dose of 50 mg/kg Retaspimycin via the tail vein. At 4, 24, and 48 h posttreatment, the animals are killed with carbon dioxide, and tumors are removed and stored at −80°C until analyzed. Four animals are used for each time point. Tumor samples are homogenized in an ice-cold, nitrogen-sparged 1:1 solution of MeOH:150 mM citrate, 0.2% (wt/vol) EDTA, and 0.2% (wt/vol) ascorbate (pH 3.0) for 1 min in an ice/water bath with a homogenizer at 17,500 rpm. Samples are centrifuged for 5 min at 4°C at 18,000 × g. The supernatants are diluted 1:1 with ice-cold, nitrogen-sparged 75 mM citrate, 0.1% (wt/vol) EDTA, and 0.1% (wt/vol) ascorbate (pH 3) containing 25 ng/mL deuterated 17-AAG as internal standard and analyzed by LC-MS/MS analysis. The standard curve is prepared for Retaspimycin, 17-AAG, and 17-AG in 1:1 MeOH:150 mM citrate, 0.2% (wt/vol) EDTA, and 0.2% (wt/vol) ascorbate (pH 3.0); diluted 1:1 with ice-cold, nitrogen-sparged 75 mM citrate, 0.1% (wt/vol) EDTA, and 0.1% (wt/vol) ascorbate (pH 3.0) containing 25 ng/mL deuterated 17-AAG as internal standard; and analyzed by LC-MS/MS[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 800.9±65.0 °C at 760 mmHg |
| Molecular Formula | C31H45N3O8 |
| Molecular Weight | 587.704 |
| Flash Point | 438.2±34.3 °C |
| Exact Mass | 587.320679 |
| PSA | 172.60000 |
| LogP | 2.62 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.587 |
| Storage condition | 2-8℃ |
|
~87% 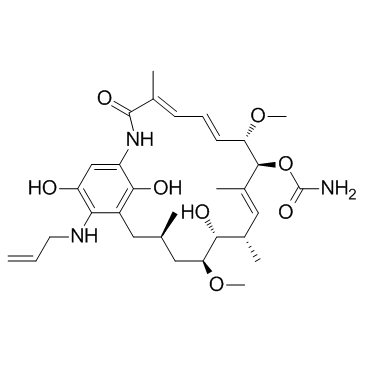
857402-23-4 |
| Literature: INFINITY PHARMACEUTICALS, INC. Patent: WO2007/2093 A2, 2007 ; Location in patent: Page/Page column 19 ; WO 2007/002093 A2 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |