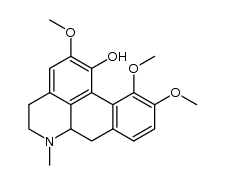
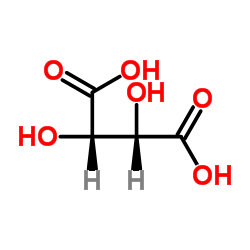
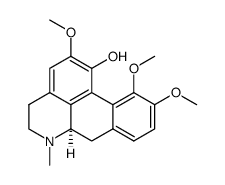
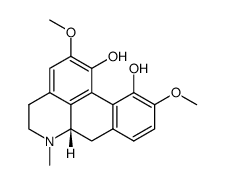
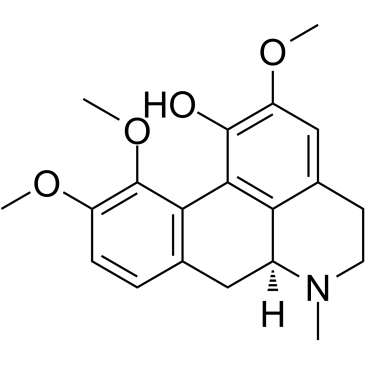
476-69-7
| Name | (+)-corydaline |
|---|---|
| Synonyms |
(S)-5,6,6a,7-Tetrahydro-2,10.11-trimethoxy-6-methyl-4H-dibenzo[de,q]quinolin-1-ol
2,10,11-Trimethoxy-6aa-aporphin-1-ol d-corydine glaucentrin glaucentrine O11-Methylcorytuberine (6aS)-2,10,11-Trimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-1-ol |
| Description | Corydine is a naturally occurring alkaloid which can be extracted from plants such as Croton echinocarpus leaves. Corydine is efficient on inhibiting reverse transcriptase (RT) activity with an IC50 of 356.8 μg/mL. Corydine displays significant in vitro anti-HIV potential, inhibiting 40% of the HIV-1 reverse transcriptase enzyme activity at a concentration of 450 μg/mL of Corydine[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 505.7±50.0 °C at 760 mmHg |
| Melting Point | 165-167ºC |
| Molecular Formula | C20H23NO4 |
| Molecular Weight | 341.401 |
| Flash Point | 259.7±30.1 °C |
| Exact Mass | 341.162720 |
| PSA | 51.16000 |
| LogP | 3.09 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.604 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
476-69-7 |
| Literature: Gadamer Archiv der Pharmazie (Weinheim, Germany), 1911 , vol. 249, p. 664 |
|
~% 
476-69-7 |
| Literature: Spaeth; Berger Chemische Berichte, 1931 , vol. 64, p. 2038,2043 |
|
~% 
476-69-7 |
| Literature: Gadamer Archiv der Pharmazie (Weinheim, Germany), 1911 , vol. 249, p. 664 |
|
~% 
476-69-7 |
| Literature: Gadamer Archiv der Pharmazie (Weinheim, Germany), 1911 , vol. 249, p. 664 |
|
~% 
476-69-7 |
| Literature: Karimova, S. U.; Israilov, I. A.; Yunusov, M. S.; Yunusov, S. Yu. Chemistry of Natural Compounds, 1980 , vol. 16, # 2 p. 177 - 180 Khimiya Prirodnykh Soedinenii, 1980 , # 2 p. 224 - 228 |
|
~% 
476-69-7 |
| Literature: Karimova, S. U.; Israilov, I. A.; Yunusov, M. S.; Yunusov, S. Yu. Chemistry of Natural Compounds, 1980 , vol. 16, # 2 p. 177 - 180 Khimiya Prirodnykh Soedinenii, 1980 , # 2 p. 224 - 228 |
|
~% 
476-69-7 |
| Literature: Gadamer Archiv der Pharmazie (Weinheim, Germany), 1911 , vol. 249, p. 664 |
|
~% 
476-69-7 |
| Literature: Gadamer Archiv der Pharmazie (Weinheim, Germany), 1911 , vol. 249, p. 664 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |