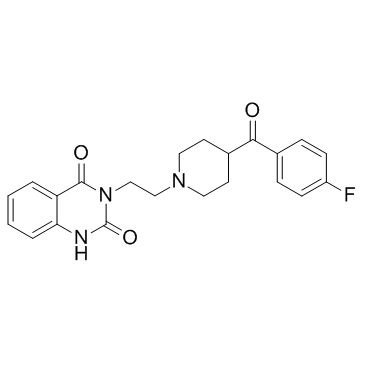
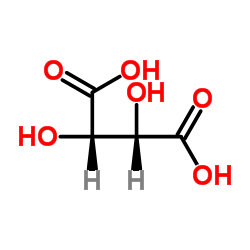
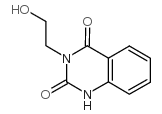
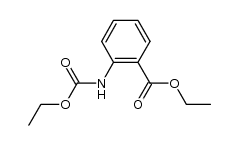
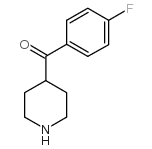
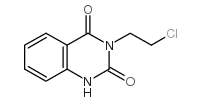
83846-83-7
| Name | Ketanserin Tartrate |
|---|---|
| Synonyms |
3-{2-[4-(4-Fluorobenzoyl)-1-piperidinyl]ethyl}-2,4(1H,3H)-quinazolinedione 2,3-dihydroxysuccinate (1:1)
EINECS 281-062-8 3-{2-[4-(4-Fluorobenzoyl)piperidin-1-yl]ethyl}quinazoline-2,4(1H,3H)-dione 2,3-dihydroxysuccinate (1:1) MFCD00084651 Ketanserin tartrate salt 2,4(1H,3H)-Quinazolinedione, 3-[2-[4-(4-fluorobenzoyl)-1-piperidinyl]ethyl]-, 2,3-dihydroxybutanedioate (1:1) (salt) Ketanserin tartrate Ketanserin tartrate,3-[2-[4-(4-Fluorobenzoyl)-1-piperidinyl]ethyl]-2,4[1H,3H]-quinazolinedionetartrate Ketanserin (tartrate) |
| Description | Ketanserin tartrate is a selective 5-HT receptor antagonist. Ketanserin tartrate also blocks hERG current (IhERG) in a concentration-dependent manner (IC50=0.11 μM). |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.11 μM (hERG current)[1] IC50: 152±23 μM (5-HT receptor)[2] |
| In Vitro | Ketanserin at 0.3 μM inhibits the voltage-dependent step current (IhERG.step) and tail current (IhERG.tail) of hERG channels with a 5-min exposure[1]. The synergistic effect observed for AA with 5-HT is, also, blocked by the 5-HT receptor blockers cyproheptadine (IC50=22.0±7 μM), Ketanserin (IC50=152±23 μM). Ketanserin (50-350 μM) inhibits the synergism by blocking the receptor in a dose-dependent manner. The IC50 value of Cyproheptadine is 22±7 μM and Ketanserin is 152±23 μM[2]. Ketanserin inhibits platelet aggregation with an IC50 of 240 (169-339) nM[3]. |
| In Vivo | Ketanserin is a 5-HT2A receptor antagonist. Ketanserin significantly reduces BDNF protein levels in numerous brain regions (CA1 and CA3 of the hippocampus, prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, shell of the nucleus accumbens and midbrain periaqueductal gray). 5-HT2A antagonist Ketanserin can significantly reduce BDNF mRNA levels in various brain regions[4]. |
| Cell Assay | The established HEK 293 cell line stably expressing hERG channels is cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum, 400 μg/mL G418. The HEK 293 cell line stably expressing recombinant human cardiac KCNQ1/KCNE1 channel current (IKs) is maintained in DMEM containing 10% foetal bovine serum and 100 μg/mL hygromycin. Cells used for electrophysiology are seeded on a glass coverslip. The mutant hERG channels are constructed, and are transiently expressed in HEK 293 cells using 10 μL of Lipofectamine 2000 with 4 μg of hERG mutant cDNA in pCDNA3 vector[1]. |
| Animal Admin | Rat[4] A total of 155 specific-pathogen-free 2-month-old male Sprague-Dawley rats, weighing 180-220 g, are used. The rats are randomly divided into the following six groups: 5-HT1A receptor agonist (8-OH-DPAT) PS group (DPAT-PS group, n=30); 5-HT1A receptor antagonist (MDL73005) PS group (MDL-PS group, n=30); 5-HT2A receptor agonist (DOI) PS group (DOI-PS group, n=30); 5-HT2A receptor antagonist (Ketanserin) PS group (Ketan-PS group, n=30); the solvent control no-stress group (0.9% physiological saline group, CON group); and the PS only group (PS group, n=30). The DPAT-PS, MDL-PS, DOI-PS, Ketan-PS and PS groups are further divided into six subgroups (n=5 each) according to the time between the stress and analysis; immediately after stress, and 0.5, 1, 2, 6 and 24 hours after stress. The CON group (n=5) receive normal feed. For the Ketan-PS group, Ketanserin, dissolved in 0.9% physiological saline, is injected intraperitoneally at 5 mg/kg at 1 hour before each stress exposure. |
| References |
| Boiling Point | 780.4ºC at 760 mmHg |
|---|---|
| Molecular Formula | C26H28FN3O9 |
| Molecular Weight | 545.514 |
| Flash Point | 425.8ºC |
| Exact Mass | 545.180969 |
| PSA | 190.23000 |
| LogP | 0.23910 |
| Appearance | solid | white |
| Storage condition | 2-8°C |
| Water Solubility | ethanol: 3.3mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S22-S36/37/39-S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | VA1411195 |
| Hazard Class | 6.1 |
|
~99% 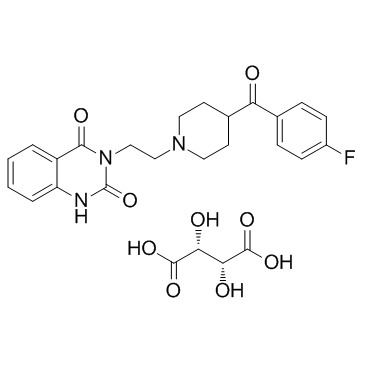
83846-83-7 |
| Literature: Fakhraian, Hossein; Heydary, Mehdi Journal of Heterocyclic Chemistry, 2014 , vol. 51, # 1 p. 151 - 156 |
|
~% 
83846-83-7 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 51, # 1 p. 151 - 156 |
|
~% 
83846-83-7 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 51, # 1 p. 151 - 156 |
|
~% 
83846-83-7 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 51, # 1 p. 151 - 156 |
|
~% 
83846-83-7 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 51, # 1 p. 151 - 156 |
|
~% 
83846-83-7 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 51, # 1 p. 151 - 156 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |