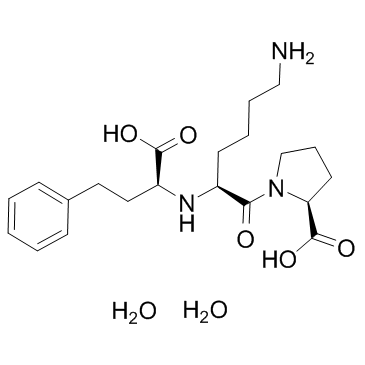
83915-83-7
| Name | lisinopril dihydrate |
|---|---|
| Synonyms |
L-Proline, N-[(1S)-1-carboxy-3-phenylpropyl]-L-lysyl-, hydrate (1:2)
(S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate N-[(1S)-1-Carboxy-3-phenylpropyl]-L-lysyl-L-proline dihydrate MFCD08064198 Lisinopril Dihydrate EINECS 278-488-1 Lisinopril diydrate Lisinopril (dihydrate) |
| Description | Lisinopril Dihydrate is angiotensin-converting enzyme inhibitor, used in treatment of hypertension, congestive heart failure, and heart attacks.Target: ACELisinopril is a potent, competitive inhibitor of angiotensin-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I (ATI) to angiotensin II (ATII). ATII regulates blood pressure and is a key component of the renin-angiotensin-aldosterone system (RAAS). Lisinopril may be used to treat hypertension and symptomatic congestive heart failure, to improve survival in certain individuals following myocardial infarction, and to prevent progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.251 g/cm3 |
|---|---|
| Boiling Point | 666.4ºC at 760 mmHg |
| Melting Point | 160ºC (Decomposes) |
| Molecular Formula | C21H35N3O7 |
| Molecular Weight | 441.52 |
| Flash Point | 356.9ºC |
| PSA | 132.96000 |
| LogP | 2.26430 |
| Index of Refraction | -45 ° (C=1, 0.25mol/L Zinc Acetate Buffer) |
| Storage condition | 2-8°C |
| Stability | Hygroscopic |
| Water Solubility | H2O: ≥10 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi,Xn |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | TW3589990 |
| WGK Germany | 3 |
| RTECS | TW3589990 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |