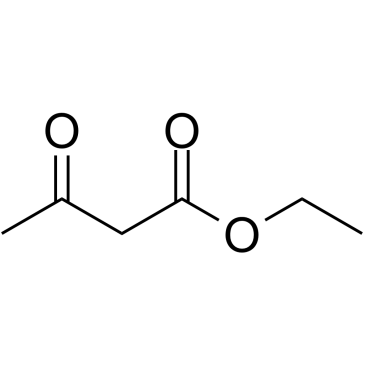
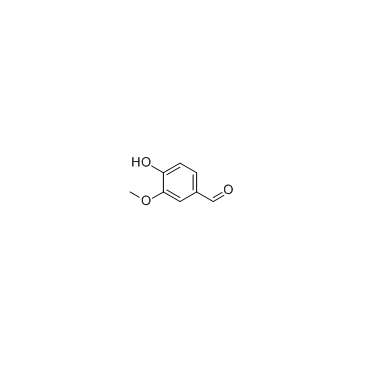
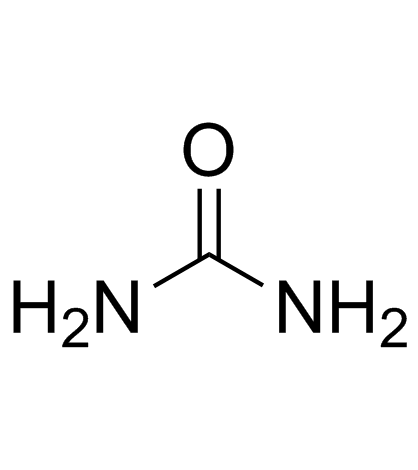
123629-42-5
| Name | ethyl 4-(4-hydroxy-3-methoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (en)5-Pyrimidinecarboxylic acid, 1,2,3,4-tetrahydro-4-(4-hydroxy-3-methoxyphenyl)-6-methyl-2-oxo-, ethyl ester (en) |
|---|---|
| Synonyms |
1,2,3,4-tetrahydro-3-quinolinamine
3-amino-1,2,3,4-tetrahydroquinoline 1,2,3,4-tetrahydroquinoline-3-amine |
| Description | gp120-IN-2 (compound 4i) is a potent HIV-1 gp120 inhibitor with an IC50 of 7.5 µM and CC50 of 112.93 µM. gp120-IN-2 shows anti-HIV-1 activity. gp120-IN-2 shows cytotoxicity in a dose dependent manner in SUP-T1 cells[1]. |
|---|---|
| Related Catalog | |
| Target |
HIV-1:7.5 μM (IC50) HIV-1:112.93 μM (CC50) |
| References |
| Molecular Formula | C15H18N2O5 |
|---|---|
| Molecular Weight | 306.31 |
| Exact Mass | 306.12200 |
| PSA | 96.89000 |
| LogP | 2.24940 |
|
~98% 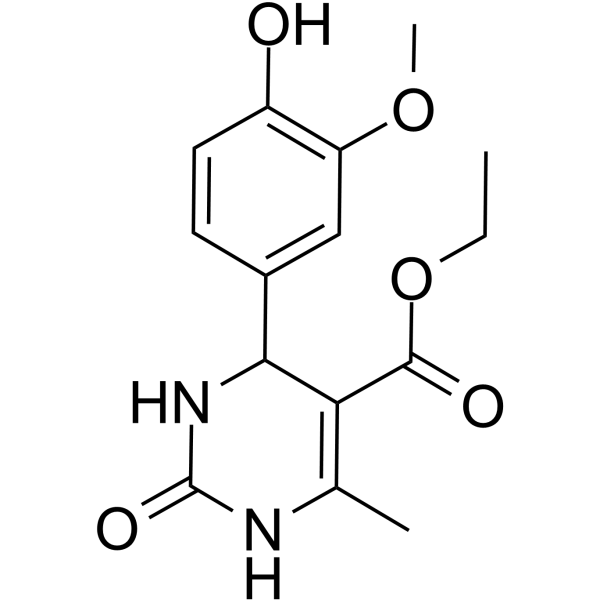
123629-42-5 |
| Literature: Alvim, Haline G. O.; De Lima, Tatiani B.; De Oliveira, Heibbe C. B.; Gozzo, Fabio C.; De MacEdo, Julio L.; Abdelnur, Patricia V.; Silva, Wender A.; Neto, Brenno A. D. ACS Catalysis, 2013 , vol. 3, # 7 p. 1420 - 1430 |