885060-09-3
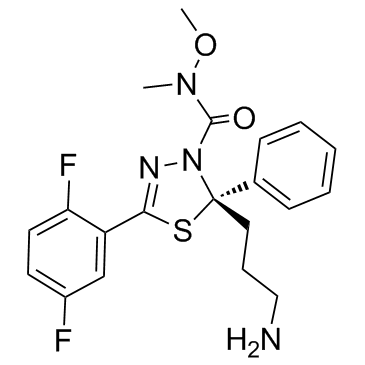
| Name | (2S)-2-(3-aminopropyl)-5-(2,5-difluorophenyl)-N-methoxy-N-methyl-2-phenyl-1,3,4-thiadiazole-3-carboxamide |
|---|---|
| Synonyms |
UNII-8A49OSO368
(2S)-2-(3-Aminopropyl)-5-(2,5-difluorophenyl)-N-methoxy-N-methyl-2-phenyl-1,3,4-thiadiazole-3(2H)-carboxamide CS-0867 ARRY 520 trifluoroacetate UNII: 8A49OSO368 (S)-2-(3-aminopropyl)-5-(2,5-difluorophenyl)-N-methoxy-N-methyl-2-phenyl-1,3,4-thiadiazole-3(2H)-carboxamide ARRY-520 Filanesib Filanesib [INN] |
| Description | Filanesib (ARRY-520) is a synthetic kinesin spindle protein (KSP) inhibitor with IC50 of 6 nM. |
|---|---|
| Related Catalog | |
| Target |
KSP:6 nM (IC50) |
| In Vitro | Filanesib (ARRY-520) retains activity in multidrug-resistant cell lines. The EC50s of Filanesib (ARRY-520) for inhibition of proliferation of HCT-15, NCI/ADR-RES and K562/ADR cells are 3.7, 14 and 4.2 nM respectively. Filanesib (ARRY-520) (10 nM) blocks a majority of cells in mitosis with the monopolar spindle structure typical of KSP inhibition[1]. Filanesib (ARRY-520) (10 nM) induces mitotic arrest as judged by both increased phosphorylation of histone H3 (pHH3) and accumulation of cyclin B1 in four cells[2]. Filanesib (ARRY-520) and Paclitaxel exhibit the same cytotoxic effect on Type I and II cells. The GI50 at 48 h for Type II EOC cells is 0.0015 μM for ARRY-520. For Type I EOC cells, the GI50 at 48 h is > 3 μM for ARRY-520[3]. Filanesib (ARRY-520) (1 nM) induces significant G2M cell cycle block in OCI-AML3 cells at 24 hours[4]. |
| In Vivo | Filanesib (ARRY-520) (10, 15, 20, 30 mg/kg, i.p.) is active in UISO-BCA-1 xenograft, and also superior to paclitaxel in mice bearing subcutaneous HT-29, HCT-116, MDA-MB-231 and A2780 xenografts. ARRY-520 is superior to docetaxel in the androgen receptor-negative prostate cancer xenograft model PC-3, and is also superior to docetaxel in the DU145 prostate xenograft model[1]. RPMI 8226 tumor xenografts are particularly sensitive to low doses of ARRY-520 (12.5 mg/kg, i.p.)[2]. ARRY-520 significantly inhibits tumor growth in HL60 and MV4-11 xenografts of SCID mice at concentrations of 27 mg/kg and 20 mg/kg, respectively[4]. |
| Cell Assay | Exponentially growing cells (0.4×106/mL) are treated with Filanesib (ARRY-520) for up to 48 hours. For combination, HL-60 and HL-60Bcl-2 cells (0.4×106/mL) are incubated with Filanesib (ARRY-520), ABT-737, or both for up to 96 hours. DMSO is used as the control agent. Apoptosis is estimated by flow cytometry measurements of phosphatidyl serine with the Annexin-V-FLUOS Staining Kit. Membrane integrity is simultaneously assessed by 7-amino-actinomycin D (7-AAD). To measure changes in the mitochondrial membrane potential (MMP), cells are loaded with CMXRos (300 nM) and MitoTracker Green (500 nM) for 1 hour at 37°C. The loss of MMP is then assessed by measuring CMXRos retention while simultaneously adjusting for mitochondrial mass. |
| Animal Admin | Subcutaneous tumor xenografts are allowed to grow to a volume of 250-350 mm3. The mice are randomized into groups of 3-4 based on tumor size, and are given a single dose of Filanesib (ARRY-520) i.p. At various time-points after administration of the drug, the mice are euthanized by CO2 inhalation and the tumors excised and placed in 10% neutral buffered formalin. The formalin-fixed tumors are processed and paraffin embedded by standard procedures. Spindle morphology is analyzed by staining tumor sections for α-tubulin, and apoptosis is analyzed by TUNEL stain. Monopolar/abnormal spindles and TUNEL positive (apoptotic) cells are counted in three ×40 fields from each sample, analyzed using algorithms developed in ImagePro software. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 511.3±60.0 °C at 760 mmHg |
| Molecular Formula | C20H22F2N4O2S |
| Molecular Weight | 420.476 |
| Flash Point | 263.0±32.9 °C |
| Exact Mass | 420.143158 |
| PSA | 96.46000 |
| LogP | 3.27 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.604 |
| Storage condition | 2-8℃ |