917389-32-3
| Name | 2-[(4S)-8-fluoro-2-[4-(3-methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-4H-quinazolin-4-yl]acetic acid |
|---|---|
| Synonyms |
Letermovir
Letermovir [INN] 1H09Y5WO1F {(4S)-8-Fluoro-2-[4-(3-methoxyphenyl)-1-piperazinyl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-3,4-dihydro-4-quinazolinyl}acetic acid AIC246 (4S)-2-{8-fluoro-2-[4-(3-methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-3,4-dihydroquinazolin-4-yl}acetic acid 2-((4S)-8-Fluoro-2-(4-(3-methoxyphenyl)piperazin-1-yl)-3-(2-methoxy-5-(trifluoromethyl)phenyl)-4H-quinazolin-4-yl)acetic acid [(S)-8-fluoro-2-[4-(3-methoxy-phenyl)-piperazin-1-yl]-3-(2-methoxy-5-trifluoromethyl-phenyl)-3,4-dihydro-quinazolin-4-yl]-acetic acid UNII-1H09Y5WO1F |
| Description | Letermovir is a novel inhibitor of CMV, which targets the viral terminase complex and remains active against virus resistant to DNA polymerase inhibitors. |
|---|---|
| Related Catalog | |
| In Vitro | AIC246 has consistent antiviral efficacy, and there is remarkable selectivity of AIC246 for human cytomegaloviruses[1]. AD169 mutant strains and designated rAIC246-1 and rAIC246-2 are highly resistant to Letermovir (AIC246), with EC50s of 5.6 nM, 1.24 μM, 0.37 μM, respectively. Letermovir inhibits HCMV replication through a specific antiviral mechanism that involves the viral gene product UL56. Letermovir inhibits HCMV replication in cell culture by interfering with the proper cleavage/packaging of HCMV progeny DNA[2]. Letermovir inhibits the current gold standard GCV by more than 400-fold with respect to EC50s (mean, 4.5 nM versus 2 μM) and by more than 2,000-fold with respect to EC90 values (mean, 6.1 nM versus 14.5 μM)[3]. Letermovir in conbination with anti-HCMV drugs causes additive antiviral effects, but there is no interaction between letermovir and anti-HIV drugs[4]. |
| In Vivo | Letermovir (10-100 mg/kg/day, p.o.) leads to a dose-dependent reduction of the HCMV titer in transplanted cells compared to that of the placebo-treated control group using the mouse xenograft model[3]. |
| Cell Assay | Briefly, 5×103 AD169-infected NHDF cells/well are seeded into the wells of 30 96-well microtiter plates. The infection is allowed to proceed under the exposure of 50 nM AIC246 (10×EC50) until a CPE developed in one or more of the compound-treated wells (indicative of resistant virus breakthrough). Noninfected and nontreated cells serve as controls on each plate. Mutant virus amplification is accomplwashed after cultures achieved maximum CPE by the passage of cell-free supernatant virus in the presence of 50 nM AIC246. The resultant AIC246-resistant progeny virus mutants are plaque purified three times by limiting dilutions in the presence of AIC246. The stability of resistance is tested by serially passaging plaque-purified viruses without selective pressure (8 to 10 times). |
| Animal Admin | Mice (18 to 25 g body weight) are anesthetized, and the Gelfoam sponges are implanted subcutaneously in the dorsoscapular area. After transplantation, mice are randomized and grouped in 10 animals per treatment group. Starting 4 h after transplantation, mice are treated once daily with letermovir for nine consecutive days. Drugs are applied per os by oral gavage. Total administration volume is 10 mL/kg. Mice are sacrificed after 9 days of treatment, and the Gelfoam implants are removed and digested with collagenase at 37°C. After 2 to 3 h, human cells are recovered by centrifugation and resuspended in GM. Subsequently, the isolated cell suspensions are serially diluted and mixed with uninfected NHDF indicator cells and PFU are determined by plaque assays as described above. Virus titers determined from isolated cells are given as PFU/mL. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 706.5±70.0 °C at 760 mmHg |
| Molecular Formula | C29H28F4N4O4 |
| Molecular Weight | 572.551 |
| Flash Point | 381.1±35.7 °C |
| Exact Mass | 572.204651 |
| PSA | 77.84000 |
| LogP | 3.47 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.601 |
| Hazard Codes | Xn |
|---|
|
~82% 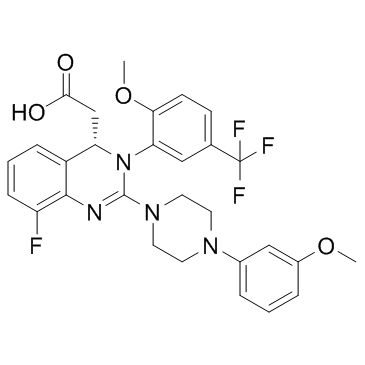
917389-32-3 |
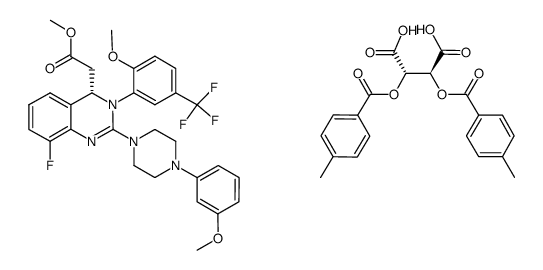
| Literature: BAYER HEALTHCARE AG Patent: WO2006/133822 A1, 2006 ; Location in patent: Page/Page column 3; 30; 31-32; 36-37 ; |
|
~% 
917389-32-3 |
| Literature: BAYER HEALTHCARE AG Patent: WO2006/133822 A1, 2006 ; Location in patent: Page/Page column 37 ; |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
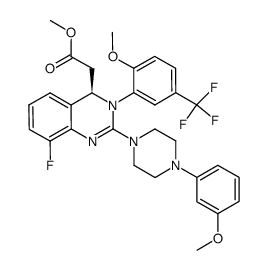

![{8-Fluoro-2-[4-(3-methoxyphenyl)-1-piperazinyl]-3-[2-methoxy-5-(t rifluoromethyl)phenyl]-3,4-dihydro-4-quinazolinyl}acetic acid structure](https://image.chemsrc.com/caspic/360/791116-51-3.png)