264233-05-8
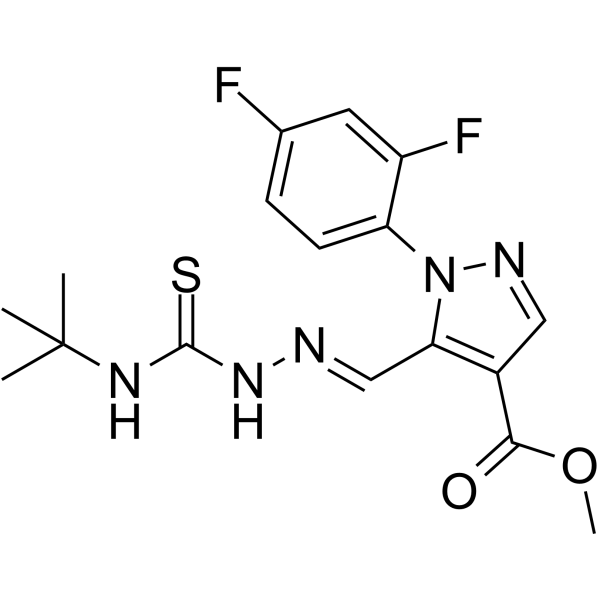
| Name | Methyl 1-(2,4-difluorophenyl)-5-[(E)-{[(2-methyl-2-propanyl)carba mothioyl]hydrazono}methyl]-1H-pyrazole-4-carboxylate |
|---|---|
| Synonyms |
1H-Pyrazole-4-carboxylic acid, 1-(2,4-difluorophenyl)-5-[(E)-[2-[[(1,1-dimethylethyl)amino]thioxomethyl]hydrazinylidene]methyl]-, methyl ester
ASTA 3746 Ciclonium bromide Methyl 1-(2,4-difluorophenyl)-5-[(E)-{[(2-methyl-2-propanyl)carbamothioyl]hydrazono}methyl]-1H-pyrazole-4-carboxylate Cicloniumbromid |
| Description | CID 2745687 acts as a specific, reversible and competitive GPR35 antagonist with a Ki of 12.8 nM[1]. |
|---|---|
| Related Catalog | |
| In Vitro | For ERK1/2 phosphorylation with 1 μM Pamoic acid as the agonist, the CID 2745687 (CID2745687) Ki is 18 nM[1]. CID 2745687 (CID-2745687) is a potent antagonist in β-arrestin-2 interaction assays only at human GPR35[2]. Using the BRET-based GPR35-β-arrestin-2 interaction assay and an EC80 concentration of Zaprinast (20 μM) as agonist, CID 2745687 behaved as a moderately potent, concentration-dependent antagonist at human GPR35 with pIC50=6.70±0.09[2]. CID 2745687 (pIC50=6.27±0.08) fully reverses the agonist action of Cromolyn disodium [2]. |
| In Vivo | CID 2745687 (CID2745687; 1 mg/kg; administrated orally every day for the last 4 weeks), a specific GPR35 antagonist, reverses Lodoxamide-mediated anti-fibrotic effects[3]. Animal Model: Six-week-old male C57BL/6 mice[3] Dosage: 1 mg/kg Administration: Oral administration, every day for 4 weeks Result: Inhibited Lodoxamide-mediated protective effects. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 493.6±55.0 °C at 760 mmHg |
| Molecular Formula | C17H19F2N5O2S |
| Molecular Weight | 395.427 |
| Flash Point | 252.3±31.5 °C |
| Exact Mass | 395.122742 |
| PSA | 119.67000 |
| LogP | 3.05 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.591 |
| Hazard Codes | Xi |
|---|