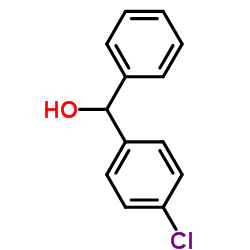
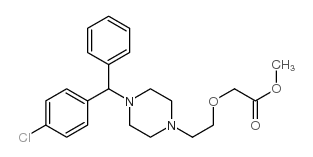
83881-52-1
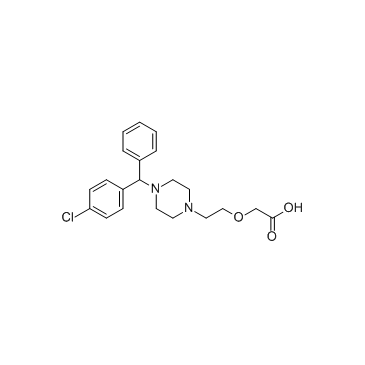
| Name | Cetirizine Dihydrochloride |
|---|---|
| Synonyms |
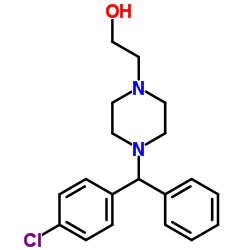
2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]acetic acid,dihydrochloride
Acetic acid, 2-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]-, hydrochloride (1:2) acide (2-{4-[(4-chlorophényl)(phényl)méthyl]pipérazin-1-yl}éthoxy)acétique dichlorhydrate Zirtek acetic acid, [2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]-, dihydrochloride EINECS 222-225-5 MFCD00941428 (2-{4-[(4-Chlorphenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)essigsäuredihydrochlorid (2-{4-[(4-Chlorophenyl)(phenyl)methyl]-1-piperazinyl}ethoxy)acetic acid dihydrochloride (2-{4-[(4-Chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)acetic acid dihydrochloride Zyrlex cetirizine dihydrochloride Cetirizine (dihydrochloride) |
| Description | Cetirizine 2Hcl, a second-generation antihistamine, is a major metabolite of hydroxyzine, and a racemic selective H1 receptor inverse agonist used in the treatment of allergies, hay fever, angioedema, and urticaria. IC50 value:Target: Histamine H1 receptorCetirizine crosses the blood-brain barrier only slightly, reducing the sedative side-effect common with older antihistamines. It has also been shown to inhibit eosinophil chemotaxis and LTB4 release. At a dosage of 20 mg, Boone et al. found that it inhibited the expression of VCAM-1 in patients with atopic dermatitis. The levorotary enantiomer of cetirizine, known as levocetirizine, is the more active form. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.237 g/cm3 |
|---|---|
| Boiling Point | 542.1ºC at 760 mmHg |
| Melting Point | 110-115ºC |
| Molecular Formula | C21H27Cl3N2O3 |
| Molecular Weight | 461.81 |
| Flash Point | 281.6ºC |
| PSA | 53.01000 |
| LogP | 3.82600 |
| Storage condition | Desiccate at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 26-36 |
| RIDADR | UN 3249 |
| WGK Germany | 3 |
| RTECS | AG0977500 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933599090 |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




![(RS)-N,N-diethyl-{2-[4-(α-phenyl-p-chloro-benzyl)piperazin-1-yl]ethoxy}-acetamide structure](https://image.chemsrc.com/caspic/252/343781-29-3.png)
![(RS)-N,N-diallyl-{2-[4-(α-phenyl-p-chloro-benzyl)piperazin-1-yl]ethoxy}-acetamide structure](https://image.chemsrc.com/caspic/397/343781-30-6.png)