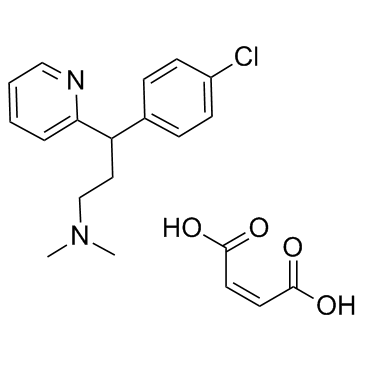
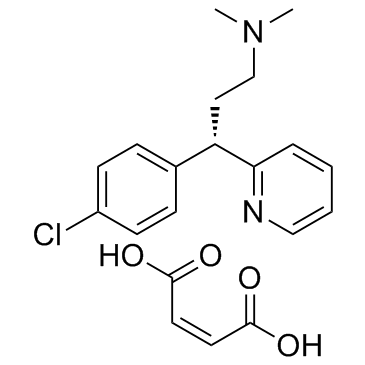
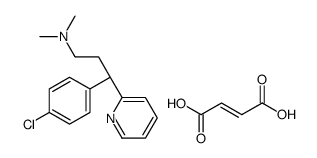
113-92-8
| Name | Chlorpheniramine maleate |
|---|---|
| Synonyms |
teldrin
CHLORPHENAMINE MALEATES BISACODYL Chlorphenamine Maleate 3-(4-chlorophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine 2-[p-Chloro-a-(2-dimethylaminoethyl)benzyl]pyridine CHLOROPHENAMINEMALEATE CHLORPHENIRAMINE-D4 MALEATE ChlorpheniramineMaleate alunex Cloropiril M.P. chlorcaps T.D. Chlorpheniramine (maleate) Haynon 3-(4-Chlorophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine (2Z)-2-butenedioate (1:1) CHLORPHENAMINE HYDROGEN MALEATE, WHO STANDARD EINECS 205-054-0 (±)-Chlorpheniramine maleate UNII:V1Q0O9OJ9Z Allergisan (±)-Chlorpheniramine 3-(4-Chlorophenyl)-N,N-dimethyl-3-(2-pyridinyl)-1-propanamine Chlortrimeton 2-pyridinepropanamine, g-(4-chlorophenyl)-N,N-dimethyl-, (2Z)-2-butenedioate (1:1) 3-(4-chlorophenyl)-N,N-dimethyl-3-pyridin-2-ylpropan-1-amine piriex Chloropheniramine maleate piriton P-CHLORO(2-DIMETHYLAMINOETHYL)BENZYLPYRIDINE MALEATE d-chlorpheniramine maleate D-2-(p-Chloro-α-(2-dimethylaminoethyl)benzyl)pyridine (+/-)-CHLORPHENIRAMINE MALEATE SALT dl-Chlorpheniramine chlorpheniramine (+/-)-CHLORPHENIRAMINE-D6 MALEATE CHLORPHENIRAMINE HYDROGEN MALEATE ibioton 2-Pyridinepropanamine Histadur c-meton 4-Chloropheniramine Puermin [3-(4-chlorophenyl)-3-(2-pyridyl)propyl]dimethylamine MFCD00069225 3-(4-Chlorophenyl)-N,N-dimethyl-3-(pyridin-2-yl)propan-1-amine (2Z)-but-2-enedioate (1:1) 2-Pyridinepropanamine, γ-(4-chlorophenyl)-N,N-dimethyl-, (2Z)-2-butenedioate (1:1) chlorphenamine Cloropiril M.P. CHLORPHENIRAMINE MALEATE REFERENCE STANDARD dexchlorpheniramine maleate 1-(N,N-Dimethylamino)-3-(p-chlorophenyl-3-a-pyridyl)propane Maleate CHLORPROPHENPYRIDAMINE MALEATE g-(4-Chlorophenyl)-N,N-dimethyl-2-pyridinepropanamine Chlorpheniramine Maleate lorphen Chlorpheniramine Maleate Salt Chlorcaps T.D. allergin g-(4-Chlorophenyl)-g-(2-pyridyl)propyldimethylamine |
| Description | Chlorpheniramine maleate is an histamine H1 receptor antagonist with IC50 of 12 nM.Target: Histamine H1 ReceptorChlorpheniramine inhibits the proliferation of MCF-7, MDA-MB 231, and Ehrlich cells in a dose-response manner, and significantly reduces the ornithine decarboxylase mRNA translation by 50%-70% at the 250 μM [1]. Chlorpheniramine displaces of [3H]pyrilamine from human histamine receptor subtype 1 expressed in CHO cells with IC50 of 66 nM. Chlorpheniramine displays antimalarial activity against CQS strain (D6) and MDR strain (Dd2) of P. falciparum with IC50 of 61.2 uM and 3.9 uM, respectively. Chlorpheniramine displays cytotoxicity against the proliferation of concanavalin A-induced murine splenic lymphocytes with IC50 of 33.4 μM [2].Oral administration of Chlorpheniramine inhibits histamine-induced mortality in guinea pigs with an ED50 of 0.17 mg/kg [3]. Oral administration of Chlorpheniramine (10 mg/kg) significantly inhibits short-duration scratching in BALB/c mice stimulated by ovalbumin active cutaneous anaphylaxis and in ICR mice subcutaneously injected with histamine, but not long-duration scratching seen in NC/Nga mice, in contrast to that of dexamethasone or tacrolimus [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 379.0±42.0 °C at 760 mmHg |
| Melting Point | 130-135 °C(lit.) |
| Molecular Formula | C20H23ClN2O4 |
| Molecular Weight | 390.861 |
| Flash Point | 183.0±27.9 °C |
| Exact Mass | 390.134644 |
| PSA | 90.73000 |
| LogP | 3.39 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.565 |
| Storage condition | -20°C Freezer |
| Water Solubility | 1-5 G/100 ML AT 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25 |
| Safety Phrases | 36/37/39-45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | US6504000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933399090 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |