67392-87-4
| Name | drospirenone |
|---|---|
| Synonyms |
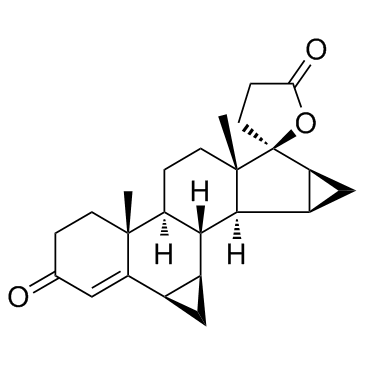
(1aR,5aR,5bS,7aS,8S,8aS,9aS,9bS,9cR,9dR)-5a,7a-Dimethyl-1,1a,5,5a,5b,6,7,7a,8a,9,9a,9b,9c,9d-tetradecahydro-3'H-spiro[cyclopropa[4,5]cyclopenta[1,2-a]cyclopropa[l]phenanthren-8,2'-furan]-3,5'(4H,4'H)-dion
arbolactone spiro[8H-cyclopropa[4,5]cyclopenta[1,2-a]cyclopropa[l]phenanthrene-8,2'(5'H)-furan]-3,5'(4H)-dione, 1,1a,3',4',5,5a,5b,6,7,7a,8a,9,9a,9b,9c,9d-hexadecahydro-5a,7a-dimethyl-, (1aR,5aR,5bS,7aS,8S,8aS,9a 6b,7b:15b,16b-Dimethylene-3-oxo-4-androstene-[17(b-1')-spiro-5']perhydrofuran-2'-one Drospirenona Drospirenone Drospirenon 6b,7b Dorospirenone (1aR,5aR,5bS,7aS,8S,8aS,9aS,9bS,9cR,9dR)-5a,7a-Dimethyl-1,1a,5,5a,5b,6,7,7a,8a,9,9a,9b,9c,9d-tetradecahydro-3'H-spiro[cyclopropa[4,5]cyclopenta[1,2-a]cyclopropa[l]phenanthrene-8,2'-furan]-3,5'(4H,4'H)-dione 1,2-Dihydrospirorenone ZK 3059 (1aR,5aR,5bS,7aS,8S,8aS,9aS,9bS,9cR,9dR)-5a,7a-diméthyl-1,1a,5,5a,5b,6,7,7a,8a,9,9a,9b,9c,9d-tétradécahydro-3'H-spiro[cyclopropa[4,5]cyclopenta[1,2-a]cyclopropa[l]phénanthrène-8,2'-furane]-3,5'(4H,4'H)-dione (1aR,5aR,5bS,7aS,8S,8aS,9aS,9bS,9cR,9dR)-5a,7a-dimethyl-1,1a,5,5a,5b,6,7,7a,8a,9,9a,9b,9c,9d-tetradecahydro-3'H-spiro[cyclopropa[4,5]cyclopenta[1,2-a]cyclopropa[l]phenanthrene-8,2'-furan]-3,5'(4H,4'H) YASMIN dihydrospirorenone |
| Description | Drospirenone(Dihydrospirorenone) is a synthetic progestin that is an analog to spironolactone.Target: Progesterone ReceptorDrospirenone is a novel progestin under clinical development that is similar to the natural hormone progesterone, combining potent progestogenic with antimineralocorticoid and antiandrogenic activities. drospirenone was devoid of glucocorticoid activity. Both progestins did not show any antiglucocorticoid action. Furthermore, drospirenone and progesterone both showed considerable antimineralocorticoid activity and weak mineralocorticoid activity [1]. the pharmacological profile of drospirenone is more closely related to that of the natural hormone progesterone than is that of any other synthetic progestogen in use today. Therefore, drospirenone is anticipated to give rise to a number of additional health benefits both for users of oral contraceptives and hormone replacement therapy recipients [2]. The combination of 17beta-estradiol and drospirenone has a positive effect on BMD and a potentially beneficial effect on lipids. Although endometrial thickness increased slightly, the safety of the endometrium was assured, as no cases of hyperplasia or cancer occurred [3].Clinical indications: Acne; Dysmenorrhea; Endometriosis; Female contraception; Folic acid deficiency; Premenstrual syndrome |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 552.2±50.0 °C at 760 mmHg |
| Melting Point | 196-200ºC |
| Molecular Formula | C24H30O3 |
| Molecular Weight | 366.493 |
| Flash Point | 241.6±30.2 °C |
| Exact Mass | 366.219482 |
| PSA | 43.37000 |
| LogP | 3.15 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.610 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360 |
| Precautionary Statements | P201-P280-P308 + P313 |
| Safety Phrases | 36/37 |
| RIDADR | NONH for all modes of transport |
| RTECS | WH1299000 |
| HS Code | 2937290090 |
| HS Code | 2937290090 |
|---|