23094-71-5
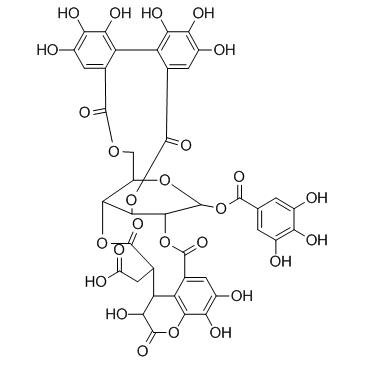
| Name | 1-O-galloyl-2,4-O-chebuloyl-3,6-O-HHDP-β-D-glucose |
|---|---|
| Synonyms |
Chebulagic acid
[(4R,5S,7R,25S,26R,29S,30S,31S)-13,14,15,18,19,20,31,35,36-Nonahydroxy-2,10,23,28,32-pentaoxo-5-[(3,4,5-trihydroxybenzoyl)oxy]-3,6,9,24,27,33-hexaoxaheptacyclo[28.7.1.0.0.0.0 .0]octatriaconta-1(38),11,13,15,17,19,21,34,36-nonaen-29-yl]acetic acid CHEBULAGIC ACID(RG) |
| Description | Chebulagic acid is a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz, on angiogenesis.target: COX-LOX [1]In vitro: Chebulagic acid can enhance the autophagy. Chebulagic acid exert anti-inflammatory and anti-infective effects. [1] [2] Chebulagic acid also show a protective effect against 1-methyl-4-phenylpyridinium (MPP+) - induce cytotoxicity which mimics the pathological symptom of Parkinson's disease. Chebulagic acid inhibit the LPS-induced upregulation of TNF-α and IL-1β in a dose- and time-dependent manner. Furthermore, LPS-activated MAPK signaling is inhibited by CA treatment in the EA.hy926 cells. [3] |
|---|---|
| Related Catalog | |
| References |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 1610.6±65.0 °C at 760 mmHg |
| Melting Point | >300℃ |
| Molecular Formula | C41H30O27 |
| Molecular Weight | 954.661 |
| Flash Point | 480.0±27.8 °C |
| Exact Mass | 954.097473 |
| PSA | 447.09000 |
| LogP | 2.25 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.876 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
| Precursor 0 | |
|---|---|
| DownStream 1 | |