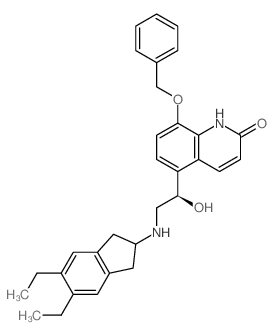
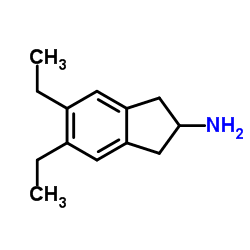
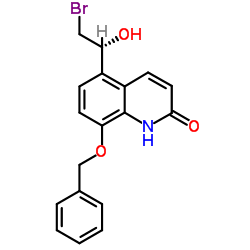
312753-06-3
| Name | indacaterol |
|---|---|
| Synonyms |
Arcapta
5-{(1R)-2-((5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino)-1-hydroxyethyl}-8-hydroxyquinolin-2(1H)-one [3H]-Indacaterol Indacaterol 5-{(1R)-2-[(5,6-Diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl}-8-hydroxy-2(1H)-quinolinone Onbrez |
| Description | Indacaterol(Onbrez; Arcapta) is an ultra-long-acting β-adrenoceptor agonist.IC50 value: Target: β-adrenoceptorIndacaterol inhibits cAMP production in Chinese hamster ovary cells stably transfected with human β2 adrenoceptors with pEC50 of 8.06. Indacaterol inhibits electrically induced contraction on the electrically stimulated guinea pig trachea in a concentration-dependent manner with pEC50 of 8.23. Indacaterol induces a concentration-dependent inotropic effect with maximal efficacy of 75% in the isolated guinea pig left atrium [1]. Indacaterol reverses the carbachol-induced contraction in a concentration-dependent manner with IC50 of 37 nM in human small airways. Indacaterol concentration dependently reverses the serotonin-induced contraction with IC50 of 10.5 nM in rat small airways. Indacaterol has the highest intrinsic efficacy of 53% in rat small airways and 73% in human small airways [2]. Indacaterol (6.7 μg/kg) inhibits 5-HT-induced bronchoconstriction with a maximal effect of 85% in the conscious guinea pig. Indacaterol (12.5 μg/kg) dose-dependently inhibits methacholine-induced bronchoconstriction with a maximal effect of 85% in the anesthetized rhesus monkey [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.27 |
|---|---|
| Molecular Formula | C24H28N2O3 |
| Molecular Weight | 392.49100 |
| Exact Mass | 392.21000 |
| PSA | 85.35000 |
| LogP | 3.53980 |
| Storage condition | 2-8°C |
|
~84% 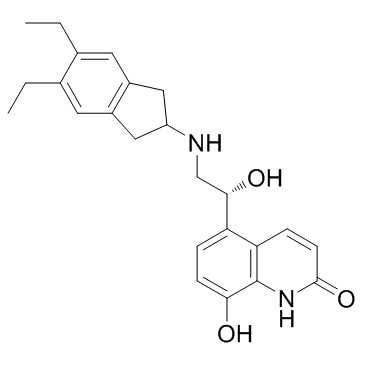
312753-06-3 |
| Literature: Baur, Francois; Beattie, David; Beer, David; Bentley, David; Bradley, Michelle; Bruce, Ian; Charlton, Steven J.; Cuenoud, Bernard; Ernst, Roland; Fairhurst, Robin A.; Faller, Bernard; Farr, David; Keller, Thomas; Fozard, John R.; Fullerton, Joe; Garman, Sheila; Hatto, Julia; Hayden, Claire; He, Handan; Howes, Colin; Janus, Diana; Jiang, Zhengjin; Lewis, Christine; Loeuillet-Ritzler, Frederique; Moser, Heinz; Reilly, John; Steward, Alan; Sykes, David; Tedaldi, Lauren; Trifilieff, Alexandre; Tweed, Morris; Watson, Simon; Wissler, Elke; Wyss, Daniel Journal of Medicinal Chemistry, 2010 , vol. 53, # 9 p. 3675 - 3684 |
|
~% 
312753-06-3 |
| Literature: WO2014/44288 A1, ; WO2014/44566 A1, ; |
|
~% 
312753-06-3 |
| Literature: WO2014/44288 A1, ; WO2014/44566 A1, ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |