651-48-9
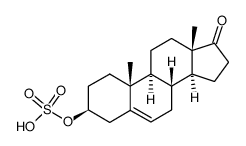
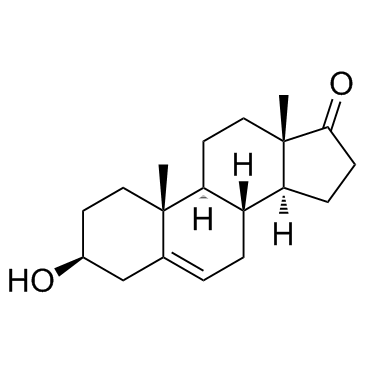
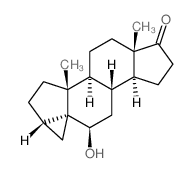
| Name | dehydroepiandrosterone sulfate |
|---|---|
| Synonyms |
Dehydroepiandrosterone 3-sulfate
dehydroepiandrosterone 3β-sulfate [3H]-Dehydroepiandrosterone 3-sulfate DHEA-sulphate dehydroepiandrosterone 3-sulphate DHEA sulfate |
| Description | Dehydroepiandrosterone sulfate, a neuroactive neurosteroid, plays a major role in brain development and aging by influencing the migration of neurons, arborization of dendrites, and formation of new synapses[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Dehydroepiandrosterone sulfate (DHEAS) selectively increased the length of neurites containing the dendritic marker MAP-2[1]. Dehydroepiandrosterone sulfate (DHEAS) has been reported to increase neuronal excitability (firing rate) when directly applied to septal-preoptic neurons[1]. Dehydroepiandrosterone (DHEA) is produced in prodigious quantities by the humanadrenal, principally as the 3-sulfoconjugate DHEA sulfate (DS) during intrauterine life[2]. Dehydroepiandrosterone sulfate (DHEAS) is generally nontoxic and lacks adverse side effects even when it is administered chronically[3]. |
| In Vivo | DHEAS chronic administration produces a dose-dependent effect on performance[3]. Animal Model: Sixty-four male Sprague-Dawley rats, approximately 90 days of age (300-400 g)[3]. Dosage: 5, 10, or 20 mg/kg. Administration: Subcutaneous injection starting 7 days post-surgery and 1 h prior to all behavioral testing. Result: Significantly effective in improving latency to reach the platform as compared to injured rats receiving vehicle. |
| References |
| Density | 1.1763 g/ml |
|---|---|
| Melting Point | 190-192 °C (lit.) |
| Molecular Formula | C19H28O5S |
| Molecular Weight | 368.49 |
| Exact Mass | 368.16600 |
| PSA | 89.05000 |
| LogP | 4.78710 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|