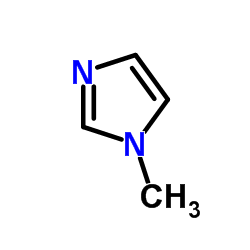
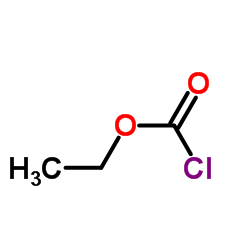
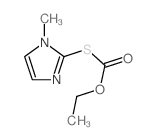
22232-54-8
| Name | carbimazole |
|---|---|
| Synonyms |
Carbimazolum
Ethyl 3-methyl-2-thioxo-2,3-dihydro-1H-imidazole-1-carboxylate EINECS 244-854-4 Carbimazol Carbimazole Neo-Thyreostat 3-Methyl-2-thioxo-2,3-dihydro-imidazol-1-carbonsaeure-aethylester 1-Methyl-3-carbethoxy-2-thioglyoxalone 1H-Imidazole-1-carboxylic acid, 2,3-dihydro-3-methyl-2-thioxo-, ethyl ester CG 1 Thyrostat ethyl 3-methyl-2-thionoimidazoline-1-carboxylate Carbethoxymethimazole 3-methyl-2-thioxo-2,3-dihydro-imidazole-1-carboxylic acid ethyl ester ethyl 3-methyl-2-sulfanylideneimidazole-1-carboxylate 3-methyl-1-ethoxycarbonylimidazoline-2-thione Carbinazole 2,3-Dihydro-3-methyl-2-thioxo-1H-imidazole-1-carboxylic Acid Ethyl Ester Athyromazole 1-Ethoxycarbonyl-3-methyl-2-thioxo-4-imidazoline MFCD00027421 Neomercazole 1-Ethoxycarbonyl-3-methyl-2-thio-4-imidazoline |
| Description | Carbimazole is an imidazole antithyroid agent. Target: OthersCarbimazole is an effective thyroid hormone inhibitor under a class of drugs known as pro-drugs. It is considered a pro-drug because it converts to methimazole after being absorbed by the body, generating an antithyroid action that works against hyperthyroidism (excessive production of thyroid hormones) and thyrotoxicosis (inflammation of the thyroid gland) [1]. Methimazole prevents the thyroid peroxidase enzyme from coupling and iodinating the tyrosine residues on thyroglobulin, hence reducing the production of the thyroid hormones T3 and T4 (thyroxine) [2].Carbimazole (CBZ) is one of the major drugs currently used for the treatment of Graves' disease. Experiments with [35S] CBZ in rats showed that the drug is so rapidly transformed to MMI after i.v. injection (within 3 min) that very little of the unchanged drug would be expected to reach the thyroid gland. The antithyroid action of CBZ in rats, therefore, can be ascribed entirely to the MMI to which it is rapidly converted [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 240.4±23.0 °C at 760 mmHg |
| Melting Point | 124°C |
| Molecular Formula | C7H10N2O2S |
| Molecular Weight | 186.232 |
| Flash Point | 99.2±22.6 °C |
| Exact Mass | 186.046295 |
| PSA | 68.25000 |
| LogP | 0.34 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.612 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Risk Phrases | R22 |
|---|---|
| Safety Phrases | S22-S36/37/39 |
| RIDADR | 3335 |
| RTECS | NJ2441000 |
| HS Code | 2922499915 |
|
~87% 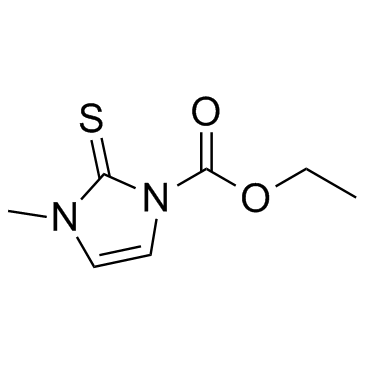
22232-54-8 |
| Literature: Das, Debasis; Roy, Gouriprasanna; Mugesh, Govindasamy Journal of Medicinal Chemistry, 2008 , vol. 51, # 22 p. 7313 - 7317 |
|
~% 
22232-54-8 |
| Literature: Journal of the Chemical Society, , p. 1103,1106 US2671088 , ; US2815349 , ; |
|
~% 
22232-54-8 |
| Literature: Journal of the Chemical Society, , p. 2387,2390 |
|
~% 
22232-54-8 |
| Literature: Journal of the Chemical Society, , p. 1103,1106 US2671088 , ; US2815349 , ; |
|
~% 
22232-54-8 |
| Literature: Journal of the Chemical Society, , p. 2387,2390 |
|
~% 
22232-54-8 |
| Literature: Journal of the Chemical Society, , p. 2387,2390 |
| Precursor 7 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |