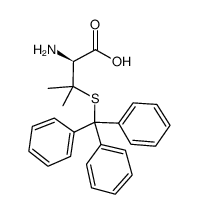
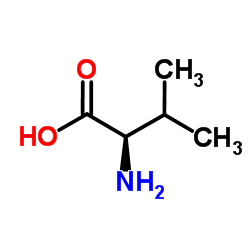
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YV9425000
-
CHEMICAL NAME :
-
Valine, 3-mercapto-, D-
-
CAS REGISTRY NUMBER :
-
52-67-5
-
LAST UPDATED :
-
199609
-
DATA ITEMS CITED :
-
47
-
MOLECULAR FORMULA :
-
C5-H11-N-O2-S
-
MOLECULAR WEIGHT :
-
149.23
-
WISWESSER LINE NOTATION :
-
SHX1&1&YZVQ -D
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
900 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
650 gm/kg/81W-I
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Musculoskeletal - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
112 mg/kg/1W-I
-
TOXIC EFFECTS :
-
Blood - agranulocytosis Skin and Appendages - dermatitis, other (after systemic exposure) Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
105 mg/kg/6W-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
400 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - other changes Blood - hemorrhage
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
150 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Cardiac - arrhythmias (including changes in conduction) Gastrointestinal - hypermotility, diarrhea
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
21 mg/kg/D
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - proteinuria Blood - leukopenia Blood - thrombocytopenia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
893 mg/kg/30W-I
-
TOXIC EFFECTS :
-
Behavioral - muscle weakness Lungs, Thorax, or Respiration - dyspnea Musculoskeletal - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
40 mg/kg/1W-I
-
TOXIC EFFECTS :
-
Blood - leukopenia Skin and Appendages - dermatitis, other (after systemic exposure) Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
482 mg/kg/19W-I
-
TOXIC EFFECTS :
-
Blood - thrombocytopenia Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6170 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2080 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4020 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
720 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
298 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3810 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3840 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - dyspnea
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4480 mg/kg/3W-I
-
TOXIC EFFECTS :
-
Liver - other changes Kidney, Ureter, Bladder - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
23400 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Blood - normocytic anemia Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
40320 mg/kg/12W-C
-
TOXIC EFFECTS :
-
Spinal Cord - other degenerative changes Autonomic Nervous System - other (direct) parasympathomimetic Nutritional and Gross Metabolic - changes in metals, not otherwise specified
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
122 gm/kg/3Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Blood - leukemia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2 gm/kg/4W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - leukemia Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
6480 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - skin and skin appendages Reproductive - Specific Developmental Abnormalities - body wall
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
9130 mg/kg
-
SEX/DURATION :
-
female 1-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1870 mg/kg
-
SEX/DURATION :
-
female 1-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other neonatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
41 gm/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating female 14 day(s) pre-mating - 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - mating performance (e.g. # sperm positive females per # females mated; # copulations per # estrus cycles) Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1750 mg/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - sex ratio
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1375 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
9130 mg/kg
-
SEX/DURATION :
-
female 1-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - skin and skin appendages Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8715 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - respiratory system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 gm/kg
-
SEX/DURATION :
-
female 15-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20160 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4) Reproductive - Effects on Newborn - other neonatal measures or effects Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10080 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other postnatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20 gm/kg
-
SEX/DURATION :
-
female 10-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
16 gm/kg
-
SEX/DURATION :
-
female 10-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3200 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4 gm/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
MUTATION DATA
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TEST SYSTEM :
-
Rodent - hamster Lung
-
DOSE/DURATION :
-
1 gm/L
-
REFERENCE :
-
MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 190,197,1987 *** REVIEWS *** TOXICOLOGY REVIEW LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,673,1971 TOXICOLOGY REVIEW PBPSDY Pharmacological and Biochemical Properties of Drug Substances. (American Pharmaceutical Assoc., 2215 Constitution Ave., NW, Washington, DC 20037) V.1- 1977- Volume(issue)/page/year: 2,465,1979 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4379 No. of Facilities: 41 (estimated) No. of Industries: 2 No. of Occupations: 11 No. of Employees: 4077 (estimated) No. of Female Employees: 1600 (estimated)
|






![[2R-[2α(R*),4β]]-5,5-dimethyl-2-[2-oxo-1-[(phenylacetyl)amino]-2-[(phenylmethyl)amino]ethyl]-4-thiazolidinecarboxylic acid structure](https://image.chemsrc.com/caspic/203/66317-00-8.png)
![[2R-[2α(R*),4β]]-2-[2-(ethylamino)-2-oxo-1-[(phenylacetyl)amino]ethyl]-5,5-dimethyl-4-thiazolidinecarboxylic acid structure](https://image.chemsrc.com/caspic/165/66317-01-9.png)
![(2R)-3-methyl-2-[(1-methylazepan-2-ylidene)amino]-3-sulfanylbutanoic acid structure](https://image.chemsrc.com/caspic/070/105099-15-8.png)
![(2R)-3-methyl-2-[(1-methylpiperidin-2-ylidene)amino]-3-sulfanylbutanoic acid structure](https://image.chemsrc.com/caspic/146/105099-13-6.png)
![(2R)-3-methyl-2-[(1-methylpyrrolidin-2-ylidene)amino]-3-sulfanylbutanoic acid structure](https://image.chemsrc.com/caspic/222/105099-11-4.png)