27262-47-1
| Name | levobupivacaine |
|---|---|
| Synonyms |
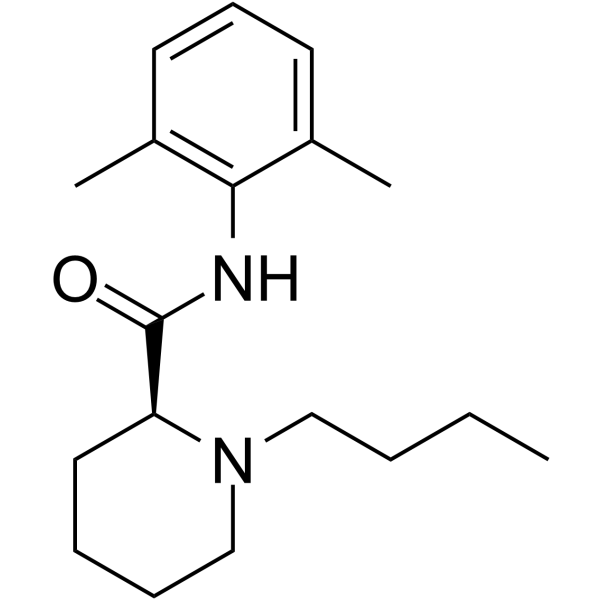
(2S)-1-Butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide
L-(-)-Bupivacaine 2',6'-Pipecoloxylidide,1-butyl-,L-(-) 1-Butyl-N-(2,6-dimethylphenyl)-piperidine-2-carboxamide (-)-Bupivacaine Levobupivacaine 1-butyl-piperidine-2-carboxylic acid-(2,6-dimethyl-anilide) (S)-(-)-Bupivacaine (S)-Bupivacaine (2S)-1-Butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide |
| Description | Levobupivacaine ((S)-(-)-Bupivacaine) is a long-acting amide local anaesthetic. Levobupivacaine exerts anaesthetic and analgesic effects through reversible blockade of neuronal sodium channel. Levobupivacaine can inhibit impulse transmission and conduction in cardiovascular and other tissues, possessing certain cardiac and CNS toxicity. Levobupivacaine is metabolized by hepatic cytochrome P450 (CYP450) enzymes in vivo. Levobupivacaine can also induce ferroptosis by miR-489-3p/SLC7A11 signaling in gastric cancer[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Sodium channels, Ferroptosis[1][2] |
| In Vitro | Levobupivacaine (0-4 mM; 24 h) does not affect the viability of GES-1 cells but inhibits the viability of HGC27 and SGC7901 cells[2]. Levobupivacaine (2 mM; 24, 48 or 72 h) enhances Erastin-induced inhibitory impact on HGC27 and SGC7901 cell viabilities; induces the levels of Fe2+, iron, and lipid ROS[2]. Levobupivacaine (2 mM; 24 h) enhances the expression of miR-489-3p in HGC27 and SGC7901 cells, increases the levels of Fe2+ and iron[2]. Cell Viability Assay[2] Cell Line: GES-1, HGC27 and SGC790 Concentration: 0, 0.5, 1, 2 and 4 mM Incubation Time: 24 h Result: Did not affect the viability of normal gastric epithelial GES-1 cell lines but inhibited the viability of HGC27 and SGC7901 cells in a dose-dependent manner. Cell Viability Assay[2] Cell Line: HGC27 and SGC7901 (incubated with 5 μM erastin) Concentration: 2 mM Incubation Time: 24, 48 or 72 h Result: Enhanced erastin-induced inhibitory impact on HGC27 and SGC7901 cell viabilities; induced the levels of Fe2+, iron, and lipid ROS. RT-PCR[2] Cell Line: HGC27 and SGC7901 (incubated with 5 μM erastin) Concentration: 2 mM Incubation Time: 24 h Result: Enhanced the expression of miR-489-3p in HGC27 and SGC7901 cells, increased the levels of Fe2+ and iron. |
| In Vivo | Levobupivacaine (40 μmol/kg; IP; once daily for 25 days) significantly inhibits SGC7901 cell growth, and enhances the lipid ROS accumulation[2]. Levobupivacaine (5 or 36 mg/kg; IP; single dosage) increases the latency to partial seizures and prevents the occurrence of generalized seizures at low dosage; reduces the latency to N-methyl-d-aspartate (NMDA)-induced seizures and increased seizure severity at high dosage[3]. Animal Model: CD1 mice (30-35 g; induced epileptic seizures by injecting with NMDA)[3] Dosage: 5 or 36 mg/kg Administration: IP; single dosage Result: Increased the latency to partial seizures and prevented the occurrence of generalized seizures at 5 mg/kg; reduced the latency to NMDA-induced seizures and increased seizure severity at 36 mg/kg. Animal Model: SCID nude mice (6-8 weeks; subcutaneously injected with 5×106 SGC7901 cells)[2] Dosage: 40 μmol/kg Administration: IP; once daily for 25 days Result: Significantly inhibited SGC7901 cell growth, and enhanced the lipid ROS accumulation. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 423.4±45.0 °C at 760 mmHg |
| Melting Point | 254ºC (dec.)(lit.) |
| Molecular Formula | C18H28N2O |
| Molecular Weight | 288.428 |
| Flash Point | 209.9±28.7 °C |
| Exact Mass | 288.220154 |
| PSA | 32.34000 |
| LogP | 3.64 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.547 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn: Harmful;T+: Very toxic; |
|---|---|
| RIDADR | UN 2811 6 |
| HS Code | 2933399090 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |