24211-30-1
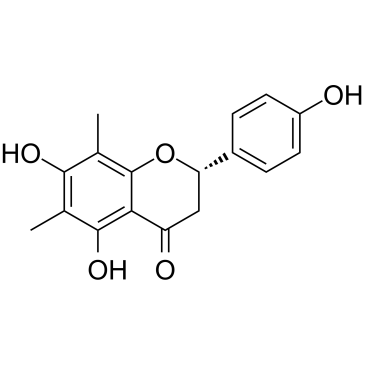
| Name | 5,7-dihydroxy-2-(4-hydroxyphenyl)-6,8-dimethyl-2,3-dihydrochromen-4-one |
|---|---|
| Synonyms |
EINECS 246-080-2
4',5,7-Trihydroxy-6,8-dimethylflavanone Farrerol 5,7-Dihydroxy-2-(4-hydroxyphenyl)-6,8-dimethylchroman-4-one 5,7-Dihydroxy-2-(4-hydroxyphenyl)-6,8-dimethyl-2,3-dihydro-4H-chromen-4-one Farresol Cyrtopterinetin 6,8-dimethyl-5,7,4'-trihydroxyflavanone MFCD00017315 matteucinol |
| Description | Farrerol is a bioactive constituent of Rhododendron, with broad activities such as anti-oxidative, anti-inflammatory, anti-tumor, neuroprotective and hepatoprotective effects[1][2][3][4][5][6]. |
|---|---|
| Related Catalog | |
| In Vitro | Farrerol observably reduces the production of inflammatory mediators including IL-1β, IL-6, TNF-α, COX-2, and iNOS in LPS-induced RAW264.7 cells via suppressing AKT, ERK1/2, JNK1/2, and NF-κB p65 phosphorylation[1]. Farrerol attenuates β-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line[2]. Farrerol inhibits angiogenesis through Akt/mTOR, Erk and Jak2/Stat3 signal pathway[3]. Farrerol overcomes the invasiveness of lung squamous cell carcinoma cells by regulating the expression of inducers of epithelial mesenchymal transition[4]. Farrerol ameliorates acetaminophen-induced hepatotoxicity via activation of Nrf2 and autophagy[6]. |
| In Vivo | Farrerol protects dopaminergic neurons in a rat model of lipopolysaccharide-induced Parkinson's disease by suppressing the activation of the AKT and NF-κB signaling pathways[5]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 583.0±50.0 °C at 760 mmHg |
| Molecular Formula | C17H16O5 |
| Molecular Weight | 300.306 |
| Flash Point | 219.7±23.6 °C |
| Exact Mass | 300.099762 |
| PSA | 86.99000 |
| LogP | 4.11 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.662 |
| Storage condition | 2~8℃ |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |