3681-99-0
| Name | Puerarin |
|---|---|
| Synonyms |
8-(β-D-Glucopyranosyl)-4',7-dihydroxyisoflavone
Puerarin std. Puerqarin Pueraria flavonoids MFCD00076007 7-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl β-D-glucopyranoside Puerain Puerarine Kakonein daidzein-8-C-glucose Purerarin 7-Hydroxy-3-(4-hydroxyphenyl)-8-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-on 7-Hydroxy-3-(4-hydroxyphényl)-8-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}-4H-chromén-4-one 7-Hydroxy-3-(4-hydroxyphenyl)-8-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one PUERARIN 7-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl b-D-glucopyranoside Puararin 7-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl-β-D-glucopyranoside |
| Description | Puerarin, an isoflavone extracted from Radix puerariae, is a 5-HT2C receptor antagonist. |
|---|---|
| Related Catalog | |
| In Vitro | Puerarin inhibits the expression of LPS-induced iNOS, COX-2 and CRP proteins and also suppresses their mRNAs from RT-PCR experiments in RAW264.7 cells. The inhibition of iNOS, COX-2 and CRP expression is due to a dose-dependent inhibition of phosphorylation and degradation of I-κB, which resulted in the reduction of p65NF-κB nuclear translocation. The effect of puerarin-mediated inhibition of LPS-induced iNOS, COX-2 and CRP expression is attributed to suppressed NF-κB activation at the transcriptional level[1]. Puerarin is a novel open-channel blocker of IK1, which may underlie the antiarrhythmic action of puerarin. Puerarin competes with barium, an open-channel blocker of IK1, to inhibit IK1 currents[2]. |
| In Vivo | Both genistein and puerarin effectively alleviate hepatic damage induced by chronic alcohol administration through potential antioxidant, anti-inflammatory, or anti apoptotic mechanisms. However, genistein is more effective than puerarin in decreasing levels of malondialdehyde (1.05±0.0947 vs. 1.28±0.213 nmol/mg pro, p< 0.05), tumor necrosis factor α (3.12±0.498 vs. 3.82±0.277 pg/mg pro, p < 0.05), interleukin-6 (1.46±0.223 vs. 1.88±0.309 pg/mg pro, p < 0.05), whereas puerarin is more effective than genistein in ameliorating serum activities or levels of alanine transaminase (35.8±3.95 vs. 42.6±6.56 U/L, p < 0.05) and low-density lipoprotein cholesterol (1.12±0.160 vs. 1.55±0.150 mmol/L, p < 0.05) [3]. Early-stage renal damages can be significantly improved by puerarin, possibly via its suppression of ICAM-1 and TNF-α expression in diabetic rat kidneys[4]. |
| Cell Assay | RAW264.7 cells are maintained at subconfluence in 95% air and 5% CO2 humidified atmosphere maintained at 37°C. The medium used for routine subculture is Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, penicillin (100 units/mL) and streptomycin (100 μg/ mL). An MTT assay is used to measure the viability of the cells after treatment with puerarin. After the supernatants are removed for nitrite determination, cells are incubated at 37°C with MTT (0.05 mg/mL) for 4 h, and the optical density is measured at 540 nm. The concentrations of puerarin are10, 20, 40 and 100 μM[1]. |
| Animal Admin | Rats: A cohort of healthy male SD rats (7 weeks old) are randomLy divided into a control group, a model group, and a puerarin treatment group with high (H), moderate (M), and low (L) dosage. Puerarin is re-suspended in 0.9% saline and is given by intra-gastric intubation at various concentrations (0.25 mg/(kg×d) for L group, 0.5 mg/(kg×d) for M group, and 1.0 mg/(kg×d) for H group) each day for 8 consecutive days. An equal volume of saline is administered to control and model rats during the same time period[4]. Mice: Forty male ICR mice (weight: 20-22 g) are acclimatized with a daily 12 h light:12 h dark cycle at 22±2 °C room temperature and 55%±5% relative humidity. After 1 week of adaption, the mice are randomLy divided into four groups with ten mice per group. Genistein and puerarin are applied to the mice in sodium carboxymethyl cellulose solution with an equimolar concentration of 0.1 M (gastric volume: 3 mL kg-1 body weight)[3]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 791.2±60.0 °C at 760 mmHg |
| Melting Point | 187-189°C |
| Molecular Formula | C21H20O9 |
| Molecular Weight | 416.38 |
| Flash Point | 281.5±26.4 °C |
| PSA | 160.82000 |
| LogP | -0.67 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.717 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | F,C |
| Risk Phrases | R11 |
| Safety Phrases | 22-24/25-45-36/37/39-26-16 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UO5216000 |
| HS Code | 2932999099 |
|
~71% 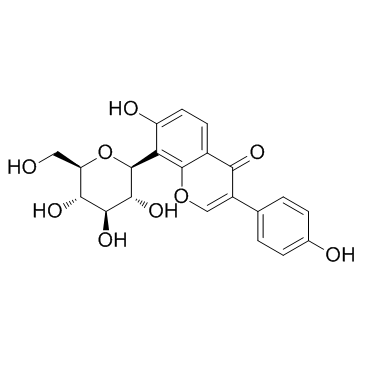
3681-99-0 |
| Literature: Kato, Eisuke; Kawabata, Jun Bioorganic and Medicinal Chemistry Letters, 2010 , vol. 20, # 15 p. 4333 - 4336 |
|
~76% 
3681-99-0 |
| Literature: Lee; Ji; Zhang Journal of Labelled Compounds and Radiopharmaceuticals, 2007 , vol. 50, # 8 p. 702 - 705 |
|
~% 
3681-99-0 |
| Literature: Lee, David Y. W.; Zhang, Wu-Yan; Karnati, Vishnu Vardhan R. Tetrahedron Letters, 2003 , vol. 44, # 36 p. 6857 - 6859 |
|
~% 
3681-99-0 |
| Literature: Park; Hakamatsuka; Noguchi; Sankawa; Ebizuka Chemical and Pharmaceutical Bulletin, 1992 , vol. 40, # 7 p. 1978 - 1980 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




