187724-61-4
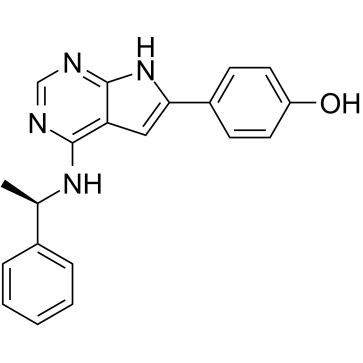
| Name | 4-[4-[[(1R)-1-phenylethyl]amino]-7H-pyrrolo[2,3-d]pyrimidin-6-yl]phenol |
|---|---|
| Synonyms |
(R)-6-(4-hydroxy-phenyl)-4-[(1-phenyl-ethyl)-amino]-7H-pyrrolo[2,3-d]pyrimidine
PKI-166 4-[(R)-(1-phenyl-ethyl)amino]-6-(4-hydroxy-phenyl)-7H-pyrrolo[2,3-d]pyrimidine 4-(R)-phenethylamino-6-(hydroxyl)phenyl-7H-pyrrolo[2.3-d]-pyrimidine (R)-4-(4-((1-phenylethyl)amino)-7H-pyrrolo[2,3-d]pyrimidin-6-yl)-phenol (R)-6-(4-hydroxy-phenyl)-4-[(1-phenylethyl)-amino]-7H-pyrrolo[2,3-d]pyrimidine |
| Description | PKI-166 is a potent, selective and orally bioavailable EGFR tyrosine kinase inhibitor, with an IC50 of 0.7 nM[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.7 nM (EGFR tyrosine kinase)[1] |
| In Vitro | Pretreatment with PKI-166 (0–0.5 μM; 1 hour) inhibits EGFR autophosphorylation in human pancreatic cancer cells[1]. PKI-166 (0.03μ M; 6 days) enhanced the cytotoxicity mediated by gemcitabine[1]. Western Blot Analysis[1] Cell Line: L3.6pl cells Concentration: 0.01 μM,0.05 μM, 0.5 μM Incubation Time: 1 hour Result: Inhibited EGFR autophosphorylation in a dose-dependent manner. Cell Cytotoxicity Assay[1] Cell Line: L3.6pl cells Concentration: 0.03 μM Incubation Time: 6 days Result: Enhanced the cytotoxicity mediated by gemcitabine. |
| In Vivo | PKI-166 (100 mg/kg; p.o.; daily; day 7-day 35 after xenograft) inhibits of pancreatic cancer growth[1]. Animal Model: Male athymic nude mice with L3.6pl cells xenograft (8–12 weeks)[1] Dosage: 100 mg/kg Administration: Oral administration; daily; from day 7 to day 35 after xenograft Result: Significantly decreased median tumor volume. |
| References |
| Molecular Formula | C20H18N4O |
|---|---|
| Molecular Weight | 330.38300 |
| Exact Mass | 330.14800 |
| PSA | 73.83000 |
| LogP | 4.57660 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | T+ |
| RIDADR | UN 2811 6.1 / PGIII |