161715-21-5
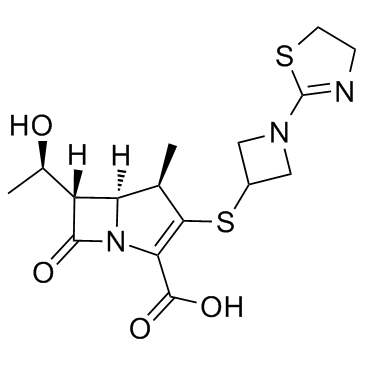
| Name | (4R,5S,6S)-3-[1-(4,5-dihydro-1,3-thiazol-2-yl)azetidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid |
|---|---|
| Synonyms |
(1R,5S,6S)-6-(1(R)-Hydroxyethyl)-1-methyl-2-(1-(2-thiazolin-2-yl)azetidin-3-ylsulfanyl)-1-carba-2-penem-3-carboxylic acid
UNII-Q2TWQ1I31U (4R,5S,6S)-3-{[1-(4,5-Dihydro-1,3-thiazol-2-yl)-3-azetidinyl]sulfanyl}-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid tebipenem 1-Azabicyclo(3.2.0)hept-2-ene-2-carboxylic acid,3-((1-(4,5-dihydro-2-thiazolyl)-3-azetidinyl)thio)-6-((1R)-1-hydroxyethyl)-4-methyl-7-oxo-,(4R,5S,6S) |
| Description | Tebipenem is an orally available carbapenem antibiotic, shows broad-spectrum activity against Gram-positive and -negative bacteria, except for Pseudomonas aeruginosa. |
|---|---|
| Related Catalog | |
| In Vitro | Tebipenem exhibits slow tight-binding inhibition at low micromolar concentrations versus the chromogenic substrate nitrocefin, and apparent Km and kcat values of 0.8 μM and 0.03 min-1, respectively[1]. Tebipenem shows potent activity against B. pseudomallei, with MIC50 and MIC90 values of both 2 mg/L[2]. Tebipenem shows good activity against S. pneumoniae, with the MIC range of ≤0.25 μg/mL in all of the S. pneumoniae isolates[3]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 624.5±65.0 °C at 760 mmHg |
| Molecular Formula | C16H21N3O4S2 |
| Molecular Weight | 383.486 |
| Flash Point | 331.5±34.3 °C |
| Exact Mass | 383.097351 |
| PSA | 144.04000 |
| LogP | -1.71 |
| Vapour Pressure | 0.0±4.1 mmHg at 25°C |
| Index of Refraction | 1.826 |
| Storage condition | 2-8℃ |