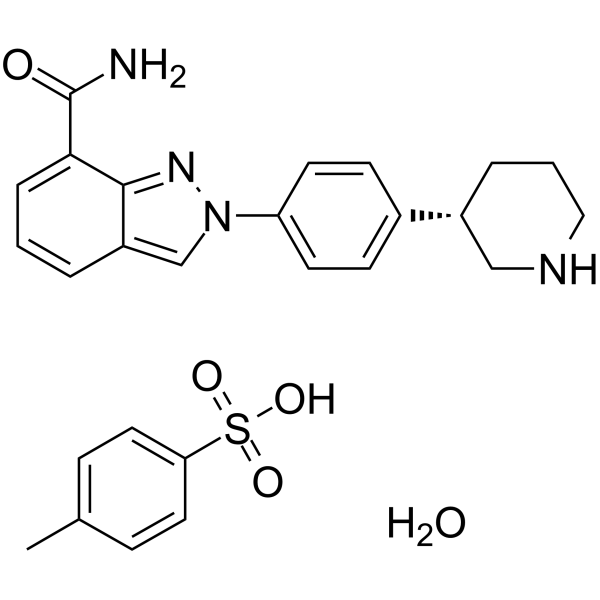
1613220-15-7
| Name | Niraparib Tosylate Monohydrate |
|---|---|
| Synonyms |
2-{4-[(3S)-3-Piperidinyl]phenyl}-2H-indazole-7-carboxamide 4-methylbenzenesulfonate hydrate (1:1:1)
2H-Indazole-7-carboxamide, 2-[4-[(3S)-3-piperidinyl]phenyl]-, 4-methylbenzenesulfonate, hydrate (1:1:1) |
| Description | Niraparib (MK-4827) tosylate hydrate is a highly potent and orally bioavailable PARP1 and PARP2 inhibitor with IC50s of 3.8 and 2.1 nM, respectively. Niraparib tosylate hydrate leads to inhibition of repair of DNA damage, activates apoptosis and shows anti-tumor activity[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
PARP-2:2.1 nM (IC50) PARP-1:3.8 nM (IC50) V-PARP:330 nM (IC50) TANK-1:570 nM (IC50) PARP-3:1300 nM (IC50) |
| In Vitro | Niraparib (MK-4827) inhibits PARP activity with EC50=4 nM and EC90=45 nM in a whole cell assay. MK-4827 inhibits proliferation of cancer cells with mutant BRCA-1 and BRCA-2 with CC50 in the 10-100 nM range. MK-4827 displays excellent PARP 1 and 2 inhibition with IC50=3.8 and 2.1 nM, respectively, and in a whole cell assay[1]. To validate that Niraparib (MK-4827) inhibits PARP in these cell lines, A549 and H1299 cells are treated with 1 μM MK-4827 for various times and measured PARP enzymatic activity using a chemiluminescent assay. The results show that Niraparib (MK-4827) inhibits PARP within 15 minutes of treatment reaching about 85% inhibition in the A549 cells at 1 h and about 55% inhibition at 1 h for the H1299 cells[2]. |
| In Vivo | Niraparib (MK-4827) is well tolerated and demonstrates efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer. Niraparib (MK-4827) is well tolerated in vivo and demonstrates efficacy as a single agent in a xenograft model of BRCA-1 deficient cancer. Niraparib (MK-4827) is characterized by acceptable pharmacokinetics in rats with plasma clearance of 28 (mL/min)/kg, very high volume of distribution (Vdss=6.9 L/kg), long terminal half-life (t1/2=3.4 h), and excellent bioavailability, F=65%[1]. Niraparib (MK-4827) enhances radiation response of p53 mutant Calu-6 tumor in both cases, with the single daily dose of 50 mg/kg being more effective than 25 mg/kg given twice daily[3]. |
| References |
| Molecular Formula | C26H30N4O5S |
|---|---|
| Molecular Weight | 510.605 |
| Exact Mass | 510.193695 |