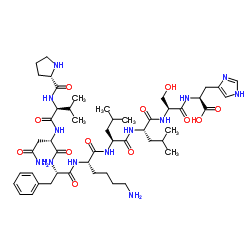
1314035-51-2
| Name | L-Prolyl-L-valyl-L-asparaginyl-L-phenylalanyl-L-lysyl-L-leucyl-L-leucyl-L-seryl-L-histidine |
|---|---|
| Synonyms |
MFCD11975008
L-Prolyl-L-valyl-L-asparaginyl-L-phenylalanyl-L-lysyl-L-leucyl-L-leucyl-L-seryl-L-histidine |
| Description | Hemopressin is a nonapeptide derived from the α1-chain of hemoglobin, is originally isolated from rat brain homogenates. Hemopressin is orally active, selective and inverse agonist of CB1 cannabinoid receptors. Hemopressin exerts antinociceptive action in inflammatory pain models[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Hemopressin causes hypotension in anesthetized rats and is metabolized in vivo and in vitro by endopeptidase 24.15 (EP24.15), neurolysin (EP24.16), and angiotensin-converting enzyme (ACE)[1]. Oral administration of Hemopressin inhibits mechanical hyperalgesia of CCI-rats up to 6h. Hemopressin treatment also decreases Egr-1 immunoreactivity (Egr-1Ir) in the superficial layer of the dorsal horn of the spinal cord of CCI rats[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 1491.3±65.0 °C at 760 mmHg |
| Molecular Formula | C50H79N13O12 |
| Molecular Weight | 1054.242 |
| Flash Point | 855.7±34.3 °C |
| Exact Mass | 1053.597168 |
| LogP | 0.60 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.566 |
| Storage condition | -20°C |