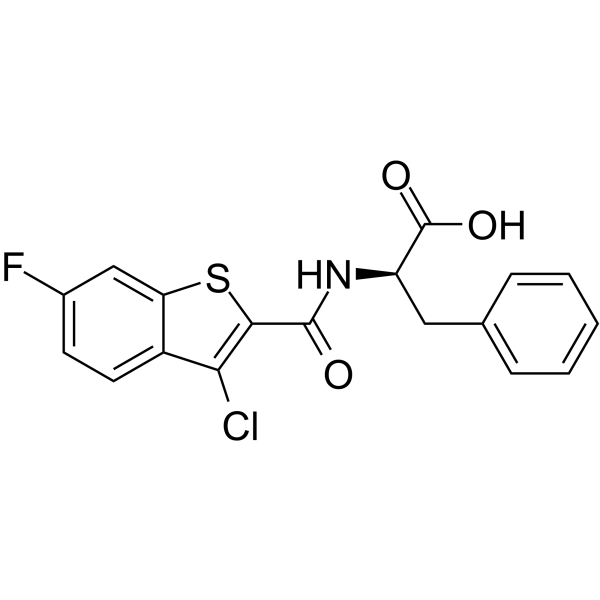
1279713-77-7
| Name | N-[(3-Chloro-6-fluoro-1-benzothiophen-2-yl)carbonyl]-D-phenylalanine |
|---|---|
| Synonyms |
N-[(3-Chloro-6-fluoro-1-benzothiophen-2-yl)carbonyl]-D-phenylalanine
TLR3-IN-1 |
| Description | CU CPT 4a is a potent inhibitor of the toll-like receptor 3 (TLR3)/double-stranded RNA (dsRNA) complex. CU CPT 4a shows dose-dependent inhibitory effects blocking Poly (I:C)-induced TLR3 activation with an IC50 of 3.44 µM[1][2]. |
|---|---|
| Related Catalog | |
| Target |
TLR3:3.44 μM (IC50, in RAW 264.7 cells) |
| In Vitro | CU-CPT4a binds to the interface of dsRNA and TLR3 and forms hydrogen bonds with Asn541[1]. |
| Cell Assay | RAW 264.7 cells are planted in 6-well plates in duplicate at 1,000,000 cells per well with 3 mL of medium (RPMI 1640 medium, supplemented with 10% FBS, Penicillin (100 U/mL) and Streptomycin (100 mg/mL)) and grown for 24 h at 37°C in a 5% CO2 humidified incubator. After 24 h, non-adherent cells and media are removed and replaced with fresh RPMI 1640 medium (3 mL/well). Two wells of adherent macrophages are treated with Poly (I:C) (15 μg/mL). One of the two wells is treated with 27 μM CU CPT 4a. One additional well is treated with only CU CPT 4a (27 μM) only. Plates are then incubated for an additional 24 h. After removal of the medium, cells are washed with PBS (3×1 mL), the 6 well plate is put on ice, then 500 μL of lysis buffer is added in each well (Lysis Buffer: 120 μL 0.5M EDTA; 12 mL Mammalian Protein Extraction Reagent, 100 μL cocktail, 0.36 mL NaCl (5 M, aqueous) ). After 5 min, the mixture is transferred into corresponding 1.5 mL tube, spun for 20 min at 13.2 K rpm in a cold room. Approximately 400 μL of supernatant are collected into new tubes, frozen at -80°C until ready for cytokine measurement[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 608.6±55.0 °C at 760 mmHg |
| Molecular Formula | C18H13ClFNO3S |
| Molecular Weight | 377.82 |
| Flash Point | 321.8±31.5 °C |
| Exact Mass | 377.028870 |
| LogP | 4.65 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Storage condition | 2-8°C |