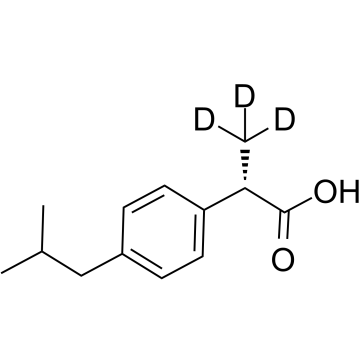
1329643-44-8
| Name | (2S)-2-(4-Isobutylphenyl)(3,3,3-2H3)propanoic acid |
|---|---|
| Synonyms | (2S)-2-(4-Isobutylphenyl)(3,3,3-2H3)propanoic acid |
| Description | (S)-(+)-Ibuprofen D3 ((S)-Ibuprofen D3) is a deuterium labeled (S)-(+)-Ibuprofen. (S)-(+)-Ibuprofen is the S(+)-enantiomer of Ibuprofen that inhibits COX-1 and COX-2 activity with IC50s of 2.1 μM and 1.6 μM. (S)-(+)-Ibuprofen has analgesic, antiinflammatory and antipyretic effects[1][2]. |
|---|---|
| Related Catalog | |
| Target |
COX-1:2.1 μM (IC50) COX-2:1.6 μM (IC50) |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 319.6±11.0 °C at 760 mmHg |
| Molecular Formula | C13H15D3O2 |
| Molecular Weight | 209.299 |
| Flash Point | 216.7±14.4 °C |
| Exact Mass | 209.149506 |
| LogP | 3.72 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.519 |