123938-86-3
| Name | GLYCOL CHITOSAN |
|---|---|
| Synonyms | MFCD00131218 |
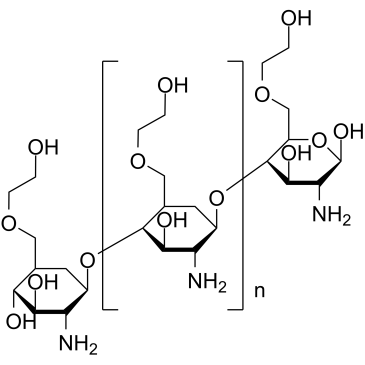
| Description | Glycol chitosan is a chitosan derivative with hydrophilic ethylene glycol branches. Glycol chitosan enhances membrane permeability and leadkage in Glycine max Harosoy 63W cells. Glycol chitosan is water-soluble, biocompatible and biodegradable[1][2][3]. Glycol chitosan inhibits E. coli, S. aureus and S. enteritidis growths with MIC values of 4 μg/mL, 32 μg/mL and <0.5 μg/mL, respectively[4]. |
|---|---|
| Related Catalog | |
| Target |
MIC: 4 μg/mL (E. coli), 32 μg/mL (S. aureus) and <0.5 μg/mL (S. enteritidis)[4] |
| In Vitro | Glycol chitosan derivatives have been successfully applied to deliver antimicrobial agents and anticancer drugs such as chemodrugs, genes, and photosensitizers (PSs), either by physical encapsulation or chemical conjugation. Glycol chitosan can be directly linked with hydrophobic drugs to generate amphiphilic compounds that can also form nanoparticles (NPs) for cell imaging and drug delivery. The use of Glycol chitosan derivatives for cell imaging and drug delivery has several advantages, including superb tumor-homing ability in the case of Glycol chitosan NPs based on enhanced permeability and retention (EPR) effect, low cytotoxicity, ease of chemical modification, great biocompatibility, and biodegradability[1]. The hydrophobic modification of Glycol chitosan is already confirmed, such as Glycol chitosan bearing a 5β-cholanic acid moiety and deoxycholic acid-Glycol chitosan, could self-assemble into nanoparticles, acting as a promising vehicle for hydrophobic drugs and genes[2]. |
| References |
| Appearance | crystalline |
|---|---|
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |