1456607-71-8
| Name | Paritaprevir dihydrate |
|---|---|
| Synonyms |
UNII:HRQ5901O78
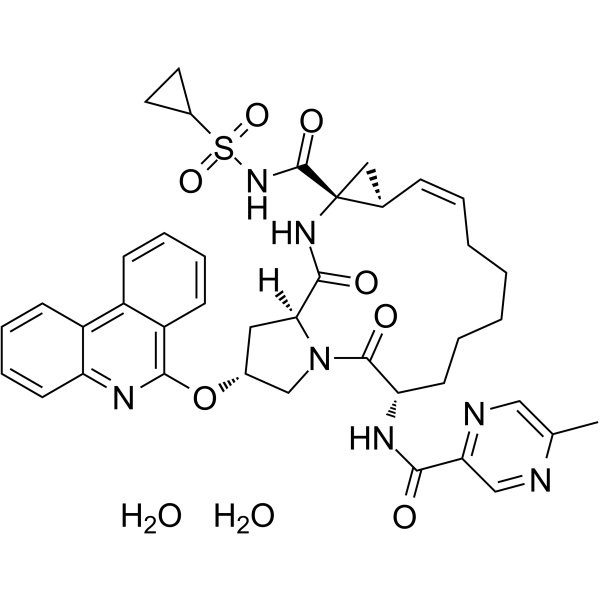
Paritaprevir dihydrate Cyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5H)-carboxamide, N-(cyclopropylsulfonyl)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydro-6-[[(5-methyl-2-pyrazinyl)carbonyl]amino]-5,16-dioxo-2-(6-phenanthridinyloxy)-, (2R,6S,12Z,13aS,14aR,16aS)-, hydrate (1:2) (2R,6S,12Z,13aS,14aR,16aS)-N-(Cyclopropylsulfonyl)-6-{[(5-methyl-2-pyrazinyl)carbonyl]amino}-5,16-dioxo-2-(6-phenanthridinyloxy)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5H)-carboxamide dihydrate |
| Description | Paritaprevir (ABT-450) dihydrate is a potent, orally active and antiviral non-structural protein 3/4A (NS3/4A) protease inhibitor with EC50s of 1 and 0.21 nM against HCV 1a and 1b, respectively. Paritaprevir dihydrate is also a SARS-CoV 3CLpro inhibitor with an IC50 of 1.31 μM. Paritaprevir dihydrate is metabolized primarily by cytochrome P450 (CYP) 3A. The plasma concentration and half-life of Paritaprevir dihydrate can be enhanced by Ritonavir (a CYP450 inhibitor)[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
EC50: 1 nM (HCV 1a), 0.21 nM (HCV 1b)[1] IC50: 1.31 μM (SARS-CoV 3CLpro)[3] |
| In Vitro | Paritaprevir has in vitro antiviral activity against HCV GT1-4 and GT6 (EC50 range, 0.09 to 19 nM), with an EC50 of 0.09 nM against GT4a[2]. |
| In Vivo | The combination of Paritaprevir, Ritonavir, Ombitasvir (an NS5A protein inhibitor), and Dasabuvir (an NS5B non-nucleoside polymerase inhibitor) with or without RBV has been approved to treat HCV genotype 1 infections[1][4]. The acute toxicity of Paritaprevir is considered to be low. Single oral doses of ≤600 mg/kg in rats and ≤100 mg/kg in dogs produces no mortality and were well tolerated. However, since Paritaprevir is administered without ritonavir as a PK enhancer, the exposures are low, especially in male rats (rat 600 mg/kg, males: Cmax 1.82 μg/mL, AUC0-24 8.89 μg·h/mL; dog 100 mg/kg, mean: Cmax 61.3 μg/mL, AUC0-24 285 μg·h/mL). |
| References |
| Molecular Formula | C40H43N7O7S.2(H2O) |
|---|---|
| Molecular Weight | 801.908 |
| Exact Mass | 801.315613 |