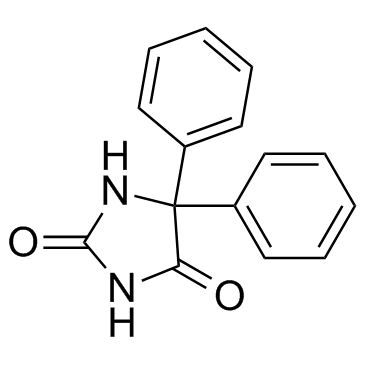
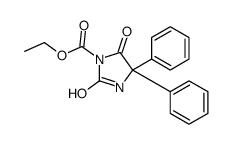
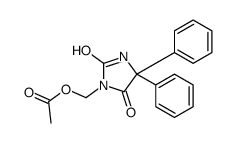
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
MU1050000
-
CHEMICAL NAME :
-
Hydantoin, 5,5-diphenyl-
-
CAS REGISTRY NUMBER :
-
57-41-0
-
LAST UPDATED :
-
199801
-
DATA ITEMS CITED :
-
121
-
MOLECULAR FORMULA :
-
C15-H12-N2-O2
-
MOLECULAR WEIGHT :
-
252.29
-
WISWESSER LINE NOTATION :
-
T5MVMV EHJ ER& ER
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
200 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - hallucinations, distorted perceptions Behavioral - ataxia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
3 mg/kg/3W
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
540 mg/kg/90D-I
-
TOXIC EFFECTS :
-
Blood - eosinophilia Lungs, Thorax, or Respiration - structural or functional change in trachea or bronchi Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
11 mg/kg/D
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Behavioral - ataxia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
31 mg/kg/4D-I
-
TOXIC EFFECTS :
-
Brain and Coverings - encephalitis Behavioral - hallucinations, distorted perceptions Behavioral - irritability
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
106 mg/kg/2W-I
-
TOXIC EFFECTS :
-
Brain and Coverings - encephalitis Behavioral - hallucinations, distorted perceptions Behavioral - ataxia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1300 mg/kg
-
TOXIC EFFECTS :
-
Blood - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
140 mg/kg/2W-I
-
TOXIC EFFECTS :
-
Liver - jaundice, other or unclassified Blood - other changes Skin and Appendages - dermatitis, other (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
15 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
15 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - excitement Behavioral - muscle contraction or spasticity
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
18 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Behavioral - excitement Behavioral - muscle contraction or spasticity
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1635 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - sleep Behavioral - altered sleep time (including change in righting reflex) Behavioral - muscle weakness
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
352 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>1500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
101 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
150 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - anticonvulsant
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
110 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
92 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
800 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
90 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
56400 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - respiratory stimulation
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - anticonvulsant
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1865 mg/kg/13W-C
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1620 mg/kg female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Reproductive - Tumorigenic effects - transplacental tumorigenesis Kidney, Ureter, Bladder - Kidney tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1620 mg/kg female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Reproductive - Tumorigenic effects - transplacental tumorigenesis Brain and Coverings - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
730 mg/kg/1Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1620 mg/kg female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Reproductive - Tumorigenic effects - transplacental tumorigenesis Brain and Coverings - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1620 mg/kg female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Reproductive - Tumorigenic effects - transplacental tumorigenesis Kidney, Ureter, Bladder - Kidney tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1500 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
52560 mg/kg/2Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1500 mg/kg/27D-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
730 mg/kg/1Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
6023 mg/kg/1Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Blood - lymphoma, including Hodgkin's disease Skin and Appendages - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
105 gm/kg/2Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5284 mg/kg/17W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - tumors Blood - lymphoma, including Hodgkin's disease
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1080 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1620 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3240 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - behavioral Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1365 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Effects on Newborn - physical Reproductive - Effects on Newborn - delayed effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
540 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
3675 mg/kg
-
SEX/DURATION :
-
female 1-35 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - skin and skin appendages Reproductive - Effects on Newborn - physical Reproductive - Effects on Newborn - delayed effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
1620 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Newborn - other postnatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
540 mg/kg
-
SEX/DURATION :
-
female 1-39 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
800 mg/kg
-
SEX/DURATION :
-
female 11-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 7-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - biochemical and metabolic
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2400 mg/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1300 mg/kg
-
SEX/DURATION :
-
female 7-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
540 mg/kg
-
SEX/DURATION :
-
female 9-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2400 mg/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
338 mg/kg
-
SEX/DURATION :
-
female 12-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 10-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 13-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 15-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1050 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other postnatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
4800 mg/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
85 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
640 mg/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating female 1-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
400 mg/kg
-
SEX/DURATION :
-
female 9-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - immune and reticuloendothelial system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
45 mg/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
630 mg/kg
-
SEX/DURATION :
-
female 8-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
58 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - cytological changes (including somatic cell genetic material)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
88 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - biochemical and metabolic
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 11-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
75 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
50 mg/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
88 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
88 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Parenteral
-
DOSE :
-
263 mg/kg
-
SEX/DURATION :
-
female 11-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
75 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
106 mg/kg
-
SEX/DURATION :
-
female 8-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
210 mg/kg
-
SEX/DURATION :
-
female 25-45 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
450 mg/kg
-
SEX/DURATION :
-
female 14-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
450 mg/kg
-
SEX/DURATION :
-
female 6-8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
330 mg/kg
-
SEX/DURATION :
-
female 8-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
Morphological transformation
-
TYPE OF TEST :
-
Sister chromatid exchange
MUTATION DATA
-
TYPE OF TEST :
-
Sister chromatid exchange
-
TEST SYSTEM :
-
Rodent - hamster Ovary
-
DOSE/DURATION :
-
1600 mg/L
-
REFERENCE :
-
EMMUEG Environmental and Molecular Mutagenesis. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.10- 1987- Volume(issue)/page/year: 10(Suppl 10),1,1987 *** REVIEWS *** IARC Cancer Review:Human Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 13,201,1977 IARC Cancer Review:Animal Limited Evidence IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,319,1987 IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 66,175,1996 IARC Cancer Review:Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 66,175,1996 IARC Cancer Review:Group 2B IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,319,1987 IARC Cancer Review:Group 2B IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 66,175,1996 TOXICOLOGY REVIEW DICPBB Drug Intelligence and Clinical Pharmacy. (POB 42435, Cincinnati, OH 45242) V.3- 1969- Volume(issue)/page/year: 8,690,1974 TOXICOLOGY REVIEW AUHPAI Australian Journal of Hospital Pharmacy. (B.R. Miller, POB 125, Heidelberg, Vic., Australia) V.1- 1971- Volume(issue)/page/year: 4(1),5,1974 TOXICOLOGY REVIEW DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. (ADIS Press International Inc., Suite B-30, Oxford Ct. Business Center, 582 Middletown Blvd., Langhorne, PA 19047) V.1- 1971- Volume(issue)/page/year: 8,354,1974 TOXICOLOGY REVIEW INTEAG Internist. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus, NJ 07094) V.1- 1960- Volume(issue)/page/year: 15,7,1974 TOXICOLOGY REVIEW EPILAK Epilepsia (New York). (Raven Press, 1185 Ave. of the Americas, New York, NY 10036) Ser.1-3: 1909-55; Ser.4: V.1- 1959- Volume(issue)/page/year: 16,159,1975 TOXICOLOGY REVIEW CLCHAU Clinical Chemistry (Winston-Salem, NC). (American Assoc. for Clinical Chemistry, 1725 K St., NW, Washington, DC 20006) V.1- 1955- Volume(issue)/page/year: 19,361,1973 TOXICOLOGY REVIEW PRSMA4 Proceedings of the Royal Society of Medicine. (New York, NY) V.1-70, 1907-77. For publisher information, see JRSMD9. Volume(issue)/page/year: 63,48,1970 TOXICOLOGY REVIEW FNSCA6 Forensic Science. (Lausanne, Switzerland) V.1-11, 1972-78. For pub lisher information, see FSINDR. Volume(issue)/page/year: 2,67,1973 TOXICOLOGY RIVIEW BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 2,442,1973 TOXICOLOGY REVIEW DMWOAX Deutsche Medizinische Wochenschrift. (Stuttgart, Fed. Rep. Ger.) V.1-104, 1875-1979. Volume(issue)/page/year: 105,719,1980 TOXICOLOGY REVIEW TJADAB Teratology, The International Journal of Abnormal Development. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1968- Volume(issue)/page/year: 19,18A,1979 TOXICOLOGY REVIEW BIREBV Biology of Reproduction. (Soc. for the Study of Reproduction, 309 W. Clark St., Champaign, IL 61820) V.1- 1969- Volume(issue)/page/year: 8,259,1973 TOXICOLOGY REVIEW 43VSAU "Phenytoin-Induced Teratology and Gingival Pathology," Hassell, T.M., et al., eds., New York, Raven Press, 1980 Volume(issue)/page/year: -,83,1980 TOXICOLOGY REVIEW JADSAY Journal of the American Dental Association. (American Dental Assoc., 211 E. Chicago Ave., Chicago, IL 60611) V.9- 1922- Volume(issue)/page/year: 99,652,1979 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 80404 No. of Facilities: 182 (estimated) No. of Industries: 2 No. of Occupations: 8 No. of Employees: 3344 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 80404 No. of Facilities: 1154 (estimated) No. of Industries: 5 No. of Occupations: 10 No. of Employees: 23400 (estimated) No. of Female Employees: 16795 (estimated)
|