28721-07-5
| Name | oxcarbazepine |
|---|---|
| Synonyms |
10,11-Dihydro-10-oxo-5h-dibenz[b,f]azepine-5-carboxamide,Oxacarbazepine
10,11-Dihydro-10-oxo-5h-dibenz[b,f]azepine-5-carboxamide Oxacarbazepine Oxecarb OXCARBAMAZEPINE Trileptal Oxcarbapezine Ocarbazepine Oxetol Aurene Oxcarbazepine 10-Oxo-10,11-dihydro-dibenzo[b,f]azepine-5-carboxylic acid amide 10,11-Dihydro-10-oxo-5H-dibenzo[b,f]azepine-5-carboxamide 10,11-Dihydro-10-oxo-5H-dibenz(b,f)azepine-5-carboxamide 10-Oxo-10,11-dihydro-5H-dibenzo[b,f]azepine-5-carboxamide EINECS 249-188-8 OXACARBAZEPINE MFCD00865307 |
| Description | Oxcarbazepine inhibits the binding of [3H]BTX to sodium channels with IC50 of 160 μM and also inhibits the influx of 22Na+ into rat brain synaptosomes with IC50 about 100 μM.Target: Sodium ChannelOxcarbazepine is an antiepileptic drug with a chemical structure similar to carbamazepine, but with different metabolism. Oxcarbazepine is rapidly reduced to 10,11-dihydro-10-hydroxy-carbazepine (monohydroxy derivative, MHD), the clinically relevant metabolite of oxcarbazepine. MHD has (S)-(+)- and the (R)-(-)-enantiomer [1]. Oxcarbazepine (oxcarb) 600 and 900 mg (2360 and 3540 mumol) was taken by 3 volunteers (2 female, 1 male; 45-67 kg; age 22-34 years) after an overnight fast. Blood, saliva and urine were collected for the next 72 h for assay of oxcarb, 10,11-dihydro-10-hydroxy-carbamazepine (OHcarb), and 10,11-dihydro-trans-10,11-dihydroxy-carbamazepine (diol). Oxcarb reached a maximum level of about 1 microgram/ml (3.93 mumol/l) within 1 h and dropped below the detection limit (0.1 microgram/ml = 0.39 mumol/l) within 3 h. The active metabolite OHcarb appeared in the blood before oxcarb and reached the higher maximum level of 7.4 microgram/ml (29 mumol/l) after 7 h [2]. Clinical indications: EpilepsyToxicity: Isolated cases of overdose with oxcarbazepine have been reported. The maximum dose taken was approximately 24,000 mg. All patients recovered with symptomatic treatment. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 457.2±55.0 °C at 760 mmHg |
| Melting Point | 215-216°C |
| Molecular Formula | C15H12N2O2 |
| Molecular Weight | 252.268 |
| Flash Point | 230.3±31.5 °C |
| Exact Mass | 252.089874 |
| PSA | 63.40000 |
| LogP | 1.44 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.662 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: ~9 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn,F |
| Risk Phrases | 22-36-20/21/22-11 |
| Safety Phrases | 16-36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HN8445000 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 6 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |



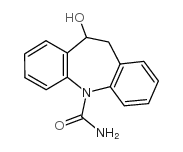
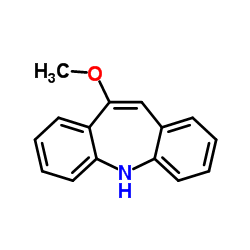
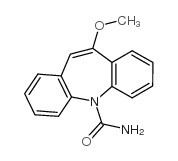
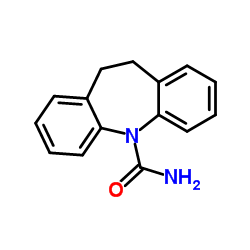

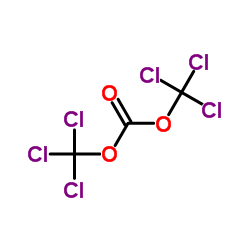
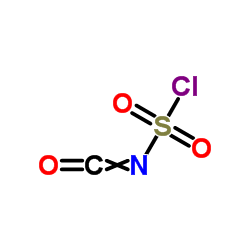
![5,11-Dihydro-10H-dibenzo[b,f]azepin-10-one structure](https://image.chemsrc.com/caspic/197/21737-58-6.png)
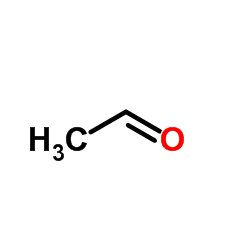
![(11-carbamoyl-5-oxo-6H-benzo[b][1]benzazepin-6-yl) acetate structure](https://image.chemsrc.com/caspic/285/113952-21-9.png)