117354-64-0
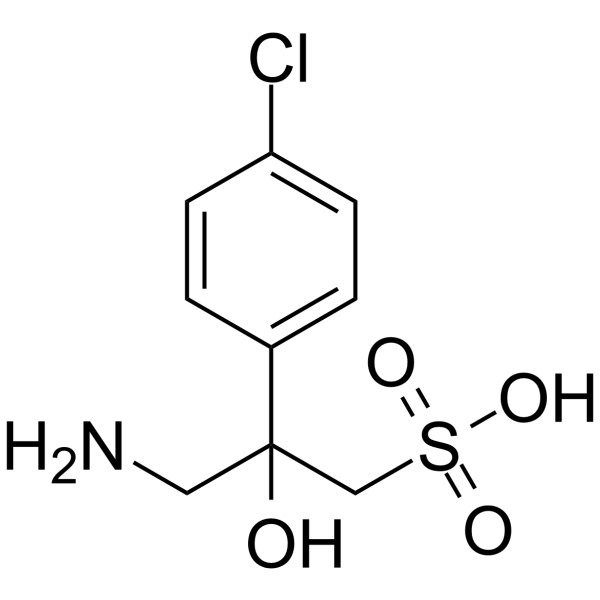
| Name | 3-amino-2-(4-chlorophenyl)-2-hydroxypropane-1-sulfonic acid |
|---|---|
| Synonyms |
2-Hydroxysaclofen
2-OH-Saclofen Dimaprit dihydrochloride MFCD00069282 |
| Description | 2-Hydroxysaclofen is a potent γ-amino-butyric-acid-B (GABAB) receptor antagonist. 2-Hydroxysaclofen can abolish nicotine-induced hypolocomotor effects and increases the antinociceptive effects. 2-Hydroxysaclofen can stimulate luteinizing hormone (LH) secretion in female rats[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
GABAB receptor[1] |
| In Vivo | 2-Hydroxysaclofen (0.25-1 mg/kg; IP; single dosage) abolishes nicotine hypolocomotor effects and increases the antinociceptive effects of nicotine[1]. 2-Hydroxysaclofen (0.3-3 mg/kg; IP; single dosage) reduces the effects of Baclofen (a GABA receptor agonist) on d-amphetamine-induced discriminative cue[2]. 2-Hydroxysaclofen (0-100 μg/2 μl; ICV; single dosage) produces a rapid elevation in the serum LH concentration in the estrogen-primed ovariectomized rat[3]. Animal Model: Male Swiss Webster mice (22-24 g; treated with 3 mg/kg nicotine)[1] Dosage: 0.25, 0.5 and 1 mg/kg Administration: IP; single dosage; after 10 or 45 min of nicotine administration Result: Abolished nicotine hypolocomotor effects at 1 mg/kg, and increased the antinociceptive effects of nicotine at 1 mg/kg. Animal Model: Male Wistar rats[2] Dosage: 0.3, 1 and 3 mg/kg Administration: IP; single dosage Result: Reduced the effects of Baclofen on d-amphetamine-induced discriminative cue. Animal Model: Female Wistar rats (8 weeks)[3] Dosage: 0, 20, 50 or 100 μg/2 μL Administration: ICV; single dosage Result: Produced a rapid elevation in the serum LH concentration in the estrogen-primed ovariectomized rat with a dose-dependent manner, and the peak LH values were observed 10 min after the injection. The effect was dose-dependent, as 0 or 20 μg of the antagonist was ineffective while a pronounced effect was observed after the injection of 50 or 100 μg. |
| References |
| Density | 1.551 g/cm3 |
|---|---|
| Melting Point | 267-269ºC |
| Molecular Formula | C9H12ClNO4S |
| Molecular Weight | 265.71400 |
| Exact Mass | 265.01800 |
| PSA | 109.00000 |
| LogP | 2.15520 |
| Index of Refraction | 1.63 |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | 26-27-36/37/39-45 |
| RIDADR | UN 1759 8/PG 3 |
| WGK Germany | 3 |